��Ŀ����
2�������£�������Һ�и���Ũ�ȹ�ϵ��ȷ���ǣ�������| A�� | pH��ȵĢ�CH3COONa ��C6H5ONa ��NaHCO3��Һ�У�c��Na+����С��ϵ���٣��ڣ��� | |
| B�� | ��ˮ�еμ�ϡ��������Һ�����ԣ�c��NH4+����c��SO42-����c��OH-��=c��H+�� | |
| C�� | ��1L0.1mol/L��NaOH��Һ��ͨ��6.6gCO2��2c��Na+��=3[c��CO32-��+c��HCO${\;}_{3}^{-}$��+c��H2CO3��] | |
| D�� | CH3COONa��Һ�м�������KNO3��ļ�����Һһ���У�c��Na+��+c��H+��=c��CH3COO-��+c��OH-�� |
���� A�������ζ���ǿ�������Σ�������Ӷ�Ӧ���������Խǿ��pH��ͬʱ��Ũ��Խ��
B��������Һ�У�c��OH-��=c��H+�������ݵ���غ��֪c��NH4+��=2c��SO42-����
C��6.6g������̼�����ʵ���Ϊ0.15mol��������̼��������Ӧ����̼�����ƣ�����̼��������Һ�е������غ��жϣ�
D�����ݻ��Һ�еĵ���غ��жϣ�
��� �⣺A��pH��ͬ�Ģ�CH3COONa ��C6H5ONa ��NaHCO3������Һ�У��ڶ�Ӧ��������������ٶ�Ӧ���������ǿ����ڵ�Ũ����С���ٵ�Ũ����������Ӳ�ˮ�⣬��������Ũ�ȴ�СΪ��c��Na+�����٣��ۣ��ڣ���A����
B����ˮ�еμ�ϡ��������Һ�����ԣ���c��OH-��=c��H+�������ݵ���غ��֪��c��NH4+��=2c��SO42-������Һ������Ũ�ȴ�СΪ����c��NH4+����c��SO42-����c��OH-��=c��H+������B��ȷ��
C����1L0.1mol/L��NaOH��Һ��ͨ��6.6gCO2��������̼�����ʵ���Ϊ��$\frac{6.6g}{44g/mol}$=0.15mol��NaOH�����ʵ���Ϊ��0.1mol/L��1L=0.1mol���������̼��������Ӧ����̼�����ƣ�����̼��������Һ�е������غ��֪��c��Na+��=c��CO32-��+c��HCO${\;}_{3}^{-}$��+c��H2CO3������C����
D��CH3COONa��Һ�м�������KNO3��ļ�����Һ�����ݵ���غ��֪��c��Na+��+c��H+��+c��K+��=c��CH3COO-��+c��OH-��+c��NO3-��������c��K+��=c��NO3-������c��Na+��+c��H+��=c��CH3COO-��+c��OH-������D��ȷ��
��ѡBD��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ�ε�ˮ��ԭ������Ӱ��Ϊ���ؼ���ע�����յ���غ㡢�����غ㼰�ε�ˮ��ԭ�����ж�����Ũ�ȴ�С�е�Ӧ�÷���������������ѧ�������Ӧ��������

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д� ͼ��������ѧϰ��ѧ��һ�ֳ��÷�����ijͬѧ���ɵ��±���������ͼ��Ӧ��ȷ���ǣ�������
ͼ��������ѧϰ��ѧ��һ�ֳ��÷�����ijͬѧ���ɵ��±���������ͼ��Ӧ��ȷ���ǣ�������| ѡ�� | x | y | z |
| A | ���� | ����� | ������Һ |
| B | ������ | ���������� | �������� |
| C | ������ | ������ | HD |
| D | ǿ����� | ǿ�� | HI |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��״���£�11.2L�ƾ�����������Ϊ0.5NA | |
| B�� | 40gNaOH������������ΪNA | |
| C�� | 1 L NaCl��Һ�У�������������Ϊ2 NA | |
| D�� | ��״���£�22.4L��������NA����ԭ�� |
| A�� | ���� | B�� | �Ȼ�����Һ | C�� | �Ȼ�ͭ��Һ | D�� | �Ȼ�þ��Һ |
| A�� | �μӼ����Ի�ɫ����Һ��Fe3+��NH4+��Cl-��SCN- | |
| B�� | pH=1����ɫ��Һ��Cu2+��Na+��Mg2+��NO3- | |
| C�� | ˮ���������c��H+��=10-13mol/L����Һ��K+��CO32-��Br-��AlO2- | |
| D�� | ��������ΪNa2CO3����Һ��K+��Cl-��NO3-��Al3+ |
| A�� | 2NaOH+H2SO4=Na2SO4+2H2OB | B�� | Cl2+H2O=HCl+HClO | ||
| C�� | CaO+H2O=C a��OH��2 | D�� | CaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2 |
;����Fe$\stackrel{ϡHCl}{��}$FeCl2��Һ ;����Fe$\stackrel{Cl_{2}}{��}$FeCl3$\stackrel{Fe��ˮ}{��}$FeCl2��Һ
;����Cl2$\stackrel{Na_{2}SO_{3}��Һ}{��}$Na2SO4��Һ ;����Cl2$\stackrel{NaOH��Һ}{��}$NaCIO��Һ$\stackrel{Na_{2}SO_{3}��Һ}{��}$Na2SO4��Һ
;����S$\stackrel{ŪHNO_{3}}{��}$ H2SO4 ;����S$\stackrel{O_{2}}{��}$SO2$\stackrel{O_{2}}{��}$SO3$\stackrel{H_{2}O}{��}$H2SO4��
| A�� | ��;���ٺ͢ڷֱ���ȡ1molFeCl2�������ϸ�����1molFe����ת��2mole- | |
| B�� | ��;���ۺֱܷ͢���ȡ1 mol Na2SO4�������ϸ�����1 molCl2����ת��2mole- | |
| C�� | ��;���ݺֱ͢���ȡ1 mol H2SO4�������ϸ�����1molS����ת��6mole- | |
| D�� | ����˵��������ȷ |
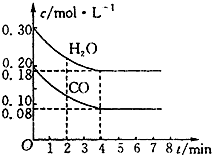 ��һ���Ϊ10L�������У�ͨ��һ������CO��H2O����850��ʱ�������·�Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g����H��0��CO��H2O��Ũ�ȱ仯��ͼ��ʾ��850��ʱ����Ũ�ȵı仯������0��4min��ƽ����Ӧ����v��CO��=0.03mol•L-1•min-1��
��һ���Ϊ10L�������У�ͨ��һ������CO��H2O����850��ʱ�������·�Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g����H��0��CO��H2O��Ũ�ȱ仯��ͼ��ʾ��850��ʱ����Ũ�ȵı仯������0��4min��ƽ����Ӧ����v��CO��=0.03mol•L-1•min-1��