��Ŀ����
����Ŀ��п������������Ԫ�أ�[Zn(NH3)4]CO3��������Եȷ��淢����Ҫ�����á�
��1��Zn2+��̬��������Ų�ʽΪ__��
��2��CO32-�Ŀռ乹��Ϊ__(����������)��[Zn(NH3)4]CO3��C��H��O��N����Ԫ�صĵ縺����С�����˳��Ϊ__��
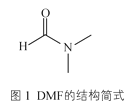
��3��ij��п����������ģ��̼����ø�Ĵ����ԣ���������к���DMF���ӡ�DMF���ӵĽṹ��ͼ1��ʾ��DMF������̼ԭ�ӹ�����ӻ�������__��1molDMF�����к�����������ĿΪ__��
��4����п��ɿ�����Zn2+��S2-�����γɵ����������ṹ�������ɡ��侧���ṹʾ��ͼ��ͼ2��ʾ����Zn2+��������������Zn2+��__����

���𰸡�[Ar]3d10��1s22s22p63s23p63d10 ƽ���������� H<C<N<O sp2��sp3 11mol��11��6.02��1023 12
��������
��1��Zn�ǵ�30��ԭ�ӣ�Zn2+�Ļ�̬��������Ų�ʽΪ[Ar]3d10��1s22s22p63s23p63d10��
��2��CO32-�ļ۲���Ӷ���Ϊ![]() �����Կռ乹����ƽ�������Σ�ͬ����Ԫ�ش�����Ԫ�صĵ縺�����������е縺��C<N<O��H�ĵ縺����С���縺��˳��Ϊ��H<C<N<O��
�����Կռ乹����ƽ�������Σ�ͬ����Ԫ�ش�����Ԫ�صĵ縺�����������е縺��C<N<O��H�ĵ縺����С���縺��˳��Ϊ��H<C<N<O��
��3��DMF�У� ��1��λ�õ�C��˫������sp2�ӻ���2��3��λ�õ�C���ǵ�������sp3�ӻ�����������������˫����һ������һ������������DMF����11NA��������
��1��λ�õ�C��˫������sp2�ӻ���2��3��λ�õ�C���ǵ�������sp3�ӻ�����������������˫����һ������һ������������DMF����11NA��������
��4����ZnS�ľ����ṹ���Կ�����ZnS�������������ܶѻ���������Zn2+��������������Zn2+��12����