��Ŀ����
����Ŀ��úȼ���ŷŵ������к���SO2�����γ����ꡢ��Ⱦ��������Чȥ��������SO2�ǻ�����������Ҫ���⡣
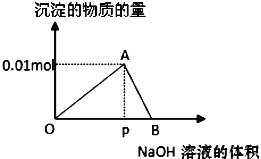
��1��˫�ϴ��SO2��NaOH��Һ![]() Na2SO3��Һ
Na2SO3��Һ
��������������Na2SO3�����ӷ���ʽΪ_________��
��˫�ϴ��SO2���ŵ�Ϊ_________��
��2��NaClO2����������SO2����NaClO2��Һ��ͨ�뺬��SO2����������Ӧ�¶�Ϊ323 K����Ӧһ��ʱ�䡣
���������շ�Ӧ�Ľ��У����ռ���Һ��pH��_________����������䡱��С������
���������NaClO���NaClO2��Ҳ�ܵõ��Ϻõ���������Ч�������յ�����SO2������NaClO�����ʵ�����NaClO2��_________����
��3��SO2�����á���������������(Na2S2O5)��ͨ������NaHSO3��������Һ���ᾧ��ˮ�Ƶá������̵����е�SO2����Na2S2O5�Ĺ������£�

�٢��з�Ӧ�Ļ�ѧ����ʽΪ___________��
��������ͨ��SO2���㣬�ᾧ��ˮ�õ���Na2S2O5�л��е���Ҫ������_______���ѧʽ����
�۹����м���Na2CO3���壬���ٴγ���SO2��Ŀ����_________��
���𰸡�2OH����SO2=SO32-��H2O ��CaO��ʹNaOH���� ��С 2 Na2CO3+2SO2+H2O=2NaHSO3+CO2 Na2SO3 �õ�NaHSO3��������Һ
��������
��1����SO2��NaOH��Ӧ������Na2SO3��H2O���ݴ�д�����ӷ���ʽ��
��˫�ϴ��SO2���ŵ�ΪCaO��ʹNaOH������
��2�������ռ���Һ�����ķ�ӦΪClO2-+2SO2+2H2O=Cl-+2SO42-+4H+����Ӧ���������ӣ�������Һ��pH��С��
�ڸ���ClO2-+2SO2+2H2O=Cl-+2SO42-+4H+��ClO-+SO2+H2O=Cl-+SO42-+2H+���з�����
��3���٢��У�pH=4.1��˵����Ӧ�����������ʣ�ֻ��ΪNaHSO3���ݴ�����д����ѧ����ʽ��
��������ͨ��SO2���㣬����ҺΪNa2SO3��NaHSO3�Ļ����ᾧ��ˮ�õ���Na2S2O5�л��е���Ҫ������Na2SO3��
��ͨ�������Ϣ��֪����������������(Na2S2O5)��ͨ������NaHSO3��������Һ���ᾧ��ˮ�Ƶá����ݴ˽��з�����
��1����SO2��NaOH��Ӧ������Na2SO3��H2O����Ӧ�����ӷ���ʽΪ2OH����SO2=SO32-��H2O��
����2OH����SO2=SO32-��H2O��
��˫�ϴ��SO2���ŵ�ΪCaO��ʹNaOH������
��Ϊ��CaO��ʹNaOH������
��2�����������շ�Ӧ�Ľ��У����ռ���Һ�����ķ�ӦΪClO2-+2SO2+2H2O=Cl-+2SO42-+4H+��������Һ��pH��С��
��Ϊ����С��
���������NaClO���NaClO2����Ӧ�����ӷ���ʽΪClO-+SO2+H2O=Cl-+SO42-+2H+������ClO2-+2SO2+2H2O=Cl-+2SO42-+4H+��Ӧ��֪�����յ�����SO2������NaClO�����ʵ�����NaClO2��2����
����2��
��3���٢��У�pH=4.1��˵����Ӧ�����������ʣ�ֻ��ΪNaHSO3����Ӧ�Ļ�ѧ����ʽΪNa2CO3+2SO2+H2O=2NaHSO3+CO2��
����Na2CO3+2SO2+H2O=2NaHSO3+CO2 ��
��������ͨ��SO2���㣬����ҺΪNa2SO3��NaHSO3�Ļ����ᾧ��ˮ�õ���Na2S2O5�л��е���Ҫ������Na2SO3��
����Na2SO3��
�������Ϣ�������ǡ���������������(Na2S2O5)��ͨ������NaHSO3��������Һ���ᾧ��ˮ�Ƶá��������м���Na2CO3���壬���ٴγ���SO2��Ŀ�ģ���Ȼ��Ϊ�����ջ��NaHSO3��������Һ��
�ʴ�Ϊ���õ�NaHSO3��������Һ��

����Ŀ�����𡢵����ס�ͭ��п�Ļ�������������Ҫ��;���ش��������⣺
��1����̬Bԭ�ӵ���ռ������ܼ��ĵ���������ͼΪ____����̬Cu+�ĺ�������Ų�ʽΪ___��
��2��������(CH3)3N������ˮ��ԭ����______��
��3�������ᣨH3PO3������Ԫ�ص�һ�ֺ����ᣬ��NaOH��Ӧֻ����NaH2PO3��Na2HPO3�����Σ���H3PO3���ӵĽṹʽΪ____��
��4��Zn2+����CN�����������꣨![]() �����γ��ȶ�����
�����γ��ȶ�����
��CN�� �ĽṹʽΪ_____��
��ÿ��������������У���ȡsp2�ӻ���ԭ����__����
��5��±��п���۵������ʾ��
ZnF2 | ZnCl2 | ZnBr2 | ZnI2 | |
�۵�/�� | 872 | 275 | 394 | 446 |
ZnF2���۵�Զ������������±��п����ԭ��Ϊ_____��
��6��������һ����ĥͿ�ϣ��������������ı��汣���㡣�����徧����ͼ1��ʾ��
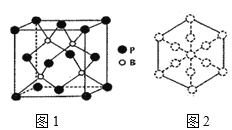
������������Խ��߷����ͶӰ��ͼ2�����ڴ���Ͻ���ʾBԭ�ӵ�ԲȦͿ��____��
����֪��������ܶ�Ϊ�� g/cm3�������ӵ�����ΪNA����B��P����Ϊ___pm���г�����ʽ���ɣ���