��Ŀ����
�о�����������SO2������������NOx��������Ҫ���壬��ش��������⣮��1���������������Ļ���������______��
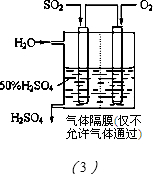
��2�����Ͱ��������������Ļ�ѧԭ���Dz��ð�ˮ����������SO2������һ�������������������ղ��ﷴӦ���õ�һ�ָ��Ϸʣ��������ֻ�����Ӫ��Ԫ�أ����ø��Ϸ��Ͽ��ܵĻ�ѧʽ______��ֻдһ�֣���
��3���������糧��ȼú�����к������ĵ������NOx���������ü����NOx��һ�������·�Ӧ��������Ⱦ��д��CH4��NO2��Ӧ�Ļ�ѧ����ʽ______��
��4��������Ӧ2NH3��g��+NO��g��+NO2��g��=2N2��g��+3H2O��g����H��0���ڵ��º��ݵ��ܱ������н��в���ƽ�⣬����˵����ȷ����______��������ţ�
A�������¶�ƽ�ⳣ�����
B������NH3��NO��NO2ת���ʱ��ƽ�ⳣ�����
C��ʹ�ô�������ƽ���ʱ�����̣���H��С
D������������壬ѹǿ���ƽ�������ƶ�
��5��ʹ�ô��������÷�Ӧ2NO��g��+2CO��g��=N2��g��+2CO2��g�������ݴ�������ij�¶��²�õ����ݻ�����ͼ��
��ǰ1S�ڵ�ƽ����Ӧ����v��CO2��=______���ڸ��¶��µ�ƽ�ⳣ��K=______��
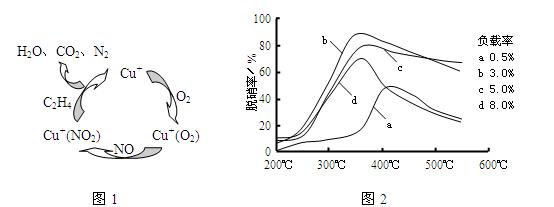
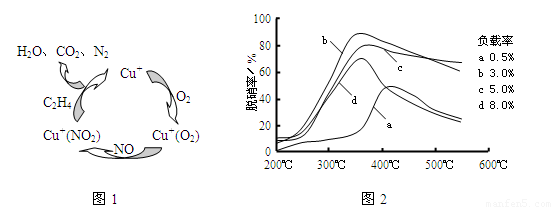
��6��Ŀǰ��ѧ�����о�����ϩ��Ϊ��ԭ����������NO��ԭ�������������������������¶ȡ������ʣ�����ɸ�д����������������Ĺ�ϵ��ͼ��Ϊ���������Ч��������ΪӦѡ�õ�������______��

���𰸡���������1��SO2�͵��������NOx���������������Ҫ���ʣ����������NOx��Ҳ������⻯ѧ������Ҫ���ʣ�
��2����ˮ����ȼú������SO2�����ɣ�NH4��2SO3 ��NH4HSO3����NH4��2SO3 ��NH4HSO3�����ᷴӦ����NH4H2PO4 ��NH4��2HPO4 ��NH4��3PO4��
��3�������NOx��һ�������·�Ӧ��������Ⱦ������N2��CO2��
��4��A�������¶ȣ�ƽ������ȷ����ƶ�������Ӧ���ȣ���������Ӧ�����ƶ���ƽ�ⳣ�����
B������NH3��ƽ��������Ӧ�����ƶ���������Ӧ��NO��NO2ת���ʱ��ƽ�ⳣ�����䣻
C��ʹ�ô������ı䷴Ӧ���̣��ӿ췴Ӧ��ƽ���ʱ�����̣���H���䣻
D�������£�����������壬��������ѹǿ���Ӧ������Ũ�Ȳ��䣬ƽ�ⲻ�ƶ���
��5��������v= ����v��CO������������֮�ȵ��ڻ�ѧ������֮����v��CO2����
����v��CO������������֮�ȵ��ڻ�ѧ������֮����v��CO2����
��ƽ�ⳣ������������Ũ��ϵ�����ݵĻ��뷴Ӧ��Ũ��ϵ�����ݵĻ�֮�ȣ�
��6�������ʸߣ������ʵͣ����˵��¶ȣ�
����⣺��1��SO2�͵��������NOx���������������Ҫ���ʣ������������������Ļ��������Ƿ�ֹ���ꡢ�⻯ѧ�����ķ������ʴ�Ϊ����ֹ���ꡢ�⻯ѧ�����ķ�����
��2����ˮ�ʼ��ԣ������������������������ˮ����ȼú������SO2�����ɣ�NH4��2SO3 ��NH4HSO3����NH4��2SO3 ��NH4HSO3�����ᷴӦ����NH4H2PO4 ��
��NH4��2HPO4 ��NH4��3PO4���ʴ�Ϊ��NH4H2PO4����NH4��2HPO4����NH4��3PO4��
��3�������NOx��һ�������·�Ӧ��������Ⱦ������������N2��CO2��ˮ����Ӧ����ʽΪ2NO2+CH4�TN2+CO2+2H2O��
�ʴ�Ϊ��2NO2+CH4�TN2+CO2+2H2O��
��4��A�������¶ȣ�ƽ������ȷ����ƶ�������Ӧ���ȣ���������Ӧ�����ƶ���ƽ�ⳣ�����A��ȷ��
B������NH3��ƽ��������Ӧ�����ƶ���������Ӧ��NO��NO2ת���ʱ��ƽ�ⳣ�����䣬��B����
C��ʹ�ô������ı䷴Ӧ���̣��ӿ췴Ӧ��ƽ���ʱ�����̣���H���䣬��C����
D�������£�����������壬��������ѹǿ���Ӧ������Ũ�Ȳ��䣬ƽ�ⲻ�ƶ�����D����
�ʴ�Ϊ��A��
��5��һ�������ķ�Ӧ����Ϊv��CO��= =
= =5.4×10-3mol/L��s��ͬһ��ѧ��Ӧͬһʱ����ڣ������ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȣ�
=5.4×10-3mol/L��s��ͬһ��ѧ��Ӧͬһʱ����ڣ������ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȣ�
����v��CO2��=v��CO��=5.4×10-3mol/L��s��
��������ʽ���2NO��g��+2CO��g��=N2��g��+2CO2��g����
��ʼŨ�ȣ�mol/L�� 10×10-4 36×10-4 0 0
�仯Ũ�ȣ�mol/L�� 9×10-4 9×10-4 4.5×10-4 9×10-4
ƽ��Ũ�ȣ�mol/L��1×10-4 27×10-4 4.5×10-4 9×10-4
����K= =5×103��mol/L�� -1
=5×103��mol/L�� -1
�ʴ�Ϊ��5.4×10-3mol/L��s��5×103��mol/L�� -1��
��6�����������ʸߣ������ʵͣ����˵��¶ȣ���ͼ��֪���ʺ�����Ϊ350�桢������3%��
�ʴ�Ϊ��350�桢������3%��
���������������������������Ϊ���壬���黷������������ʽ��д����ѧƽ�⡢��ѧ��Ӧ���ʵȣ���Ŀ�Ѷ��еȣ�����ע��������ݴ���������ͼ�����������
��2����ˮ����ȼú������SO2�����ɣ�NH4��2SO3 ��NH4HSO3����NH4��2SO3 ��NH4HSO3�����ᷴӦ����NH4H2PO4 ��NH4��2HPO4 ��NH4��3PO4��
��3�������NOx��һ�������·�Ӧ��������Ⱦ������N2��CO2��
��4��A�������¶ȣ�ƽ������ȷ����ƶ�������Ӧ���ȣ���������Ӧ�����ƶ���ƽ�ⳣ�����
B������NH3��ƽ��������Ӧ�����ƶ���������Ӧ��NO��NO2ת���ʱ��ƽ�ⳣ�����䣻
C��ʹ�ô������ı䷴Ӧ���̣��ӿ췴Ӧ��ƽ���ʱ�����̣���H���䣻
D�������£�����������壬��������ѹǿ���Ӧ������Ũ�Ȳ��䣬ƽ�ⲻ�ƶ���
��5��������v=
 ����v��CO������������֮�ȵ��ڻ�ѧ������֮����v��CO2����
����v��CO������������֮�ȵ��ڻ�ѧ������֮����v��CO2������ƽ�ⳣ������������Ũ��ϵ�����ݵĻ��뷴Ӧ��Ũ��ϵ�����ݵĻ�֮�ȣ�
��6�������ʸߣ������ʵͣ����˵��¶ȣ�
����⣺��1��SO2�͵��������NOx���������������Ҫ���ʣ������������������Ļ��������Ƿ�ֹ���ꡢ�⻯ѧ�����ķ������ʴ�Ϊ����ֹ���ꡢ�⻯ѧ�����ķ�����
��2����ˮ�ʼ��ԣ������������������������ˮ����ȼú������SO2�����ɣ�NH4��2SO3 ��NH4HSO3����NH4��2SO3 ��NH4HSO3�����ᷴӦ����NH4H2PO4 ��
��NH4��2HPO4 ��NH4��3PO4���ʴ�Ϊ��NH4H2PO4����NH4��2HPO4����NH4��3PO4��
��3�������NOx��һ�������·�Ӧ��������Ⱦ������������N2��CO2��ˮ����Ӧ����ʽΪ2NO2+CH4�TN2+CO2+2H2O��
�ʴ�Ϊ��2NO2+CH4�TN2+CO2+2H2O��
��4��A�������¶ȣ�ƽ������ȷ����ƶ�������Ӧ���ȣ���������Ӧ�����ƶ���ƽ�ⳣ�����A��ȷ��
B������NH3��ƽ��������Ӧ�����ƶ���������Ӧ��NO��NO2ת���ʱ��ƽ�ⳣ�����䣬��B����
C��ʹ�ô������ı䷴Ӧ���̣��ӿ췴Ӧ��ƽ���ʱ�����̣���H���䣬��C����
D�������£�����������壬��������ѹǿ���Ӧ������Ũ�Ȳ��䣬ƽ�ⲻ�ƶ�����D����
�ʴ�Ϊ��A��
��5��һ�������ķ�Ӧ����Ϊv��CO��=
 =
= =5.4×10-3mol/L��s��ͬһ��ѧ��Ӧͬһʱ����ڣ������ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȣ�
=5.4×10-3mol/L��s��ͬһ��ѧ��Ӧͬһʱ����ڣ������ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȣ�����v��CO2��=v��CO��=5.4×10-3mol/L��s��
��������ʽ���2NO��g��+2CO��g��=N2��g��+2CO2��g����
��ʼŨ�ȣ�mol/L�� 10×10-4 36×10-4 0 0
�仯Ũ�ȣ�mol/L�� 9×10-4 9×10-4 4.5×10-4 9×10-4
ƽ��Ũ�ȣ�mol/L��1×10-4 27×10-4 4.5×10-4 9×10-4
����K=
 =5×103��mol/L�� -1
=5×103��mol/L�� -1�ʴ�Ϊ��5.4×10-3mol/L��s��5×103��mol/L�� -1��
��6�����������ʸߣ������ʵͣ����˵��¶ȣ���ͼ��֪���ʺ�����Ϊ350�桢������3%��
�ʴ�Ϊ��350�桢������3%��
���������������������������Ϊ���壬���黷������������ʽ��д����ѧƽ�⡢��ѧ��Ӧ���ʵȣ���Ŀ�Ѷ��еȣ�����ע��������ݴ���������ͼ�����������

��ϰ��ϵ�д�
�����Ŀ