��Ŀ����
��.��˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ������е�5����Ӧ(�ɰ�����HCl��ˮ�Ʊ�NH4C1ˮ��Һ)�����жϷ�Ӧ�ܵķ�Ӧ�ȣ���H�� ��
�� NH3(g) + HCl(g) = NH4Cl(s) ��H=��176kJ��mol�C1
�� NH3(g) + H2O(l) = NH3(aq) ��H=��35.1 kJ��mol�C1
�� HCl(g) + H2O(l) = HCl(aq) ��H=��72.3 kJ��mol�C1
�� NH4C1(s) + H2O(1) = NH4C1(aq)
�� NH3(aq) + HCl(aq) = NH4C1(aq) ��H= ��52.3 kJ��mol�C1
��. N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע������H2��O2��������Na2CO3��ɵ�ȼ�ϵ�أ����õ�ⷨ�Ʊ�N2O5��װ����ͼ��ʾ������YΪCO2��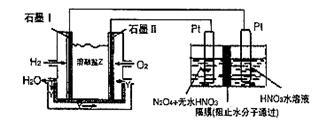
д��ʯīI�缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ ��
�ڵ���������N2O3�ĵ缫��ӦʽΪ ��
��.����������SO2����������������NOx���������ǻ�����ѧ�о����ȵ㡣
���������������Ļ��������� ��
(2)Ŀǰ����ѧ�������о�һ������ϩ��Ϊ��ԭ����������NO��ԭ��������������ʾ��ͼ����ͼ1�����������¶ȡ������ʣ�����ɸ�д����������������Ĺ�ϵ��ͼ2��ʾ��
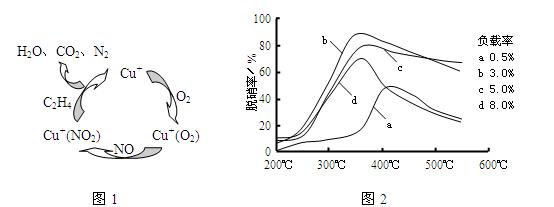
��д��������ԭ���ܷ�Ӧ�Ļ�ѧ����ʽ�� ����Ϊ�ﵽ�������Ч����Ӧ��ȡ�������� ��
��H��+16.3 kJ��mol�C1��
��3��H2+CO![]() ��2e��=CO2+H2O��������N2O4+2HNO3��2e��=2N??2O5+2H+��
��2e��=CO2+H2O��������N2O4+2HNO3��2e��=2N??2O5+2H+��
�ŷ�ֹ����ķ�������6NO��3O2��2C2H4![]() 3N2��4CO2��4H2O������������ƽ��ʽҲ�ԣ�����350�桢������3%��
3N2��4CO2��4H2O������������ƽ��ʽҲ�ԣ�����350�桢������3%��
����:
��

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�