��Ŀ����
��֪H2(g)��Br2(l)��2HBr(g) ��H����72 kJ/mol������1mol Br2(l)��Ҫ���յ�����Ϊ30kJ����������������±���
| | H2(g) | Br2(g) | HBr(g) |
| 1mol�����еĻ�ѧ������ʱ��Ҫ���յ�����/kJ | 436 | a | 369 |
A��Br2(l)��Br2(g) ��S��0
B��Br2(l)��Br2(g) ��H����30 kJ/mol
C��H��H���ļ���Ϊ436 kJ
D��a=200
D
�������������A��Br2(l)��Br2(g)�����Ҷ�����S��0��B��Br2(l)��Br2(g)�����ȷ�Ӧ����H����30 kJ/mol��C��H��H���ļ���Ϊ436 kJ/mol��D����ݼ��ܼ��㣺369��2��436��a��30=72�����a=200����ȷ��
���㣺�����Ȼ�ѧ����ʽ�ĺ��弰���ܵļ��㡣
���������ݷ�Ӧ�ȵ��ڷ�Ӧ��ļ��ܼ�ȥ������ļ��ܣ������ѶȲ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
�����Ŀ
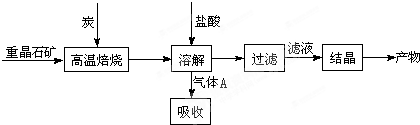
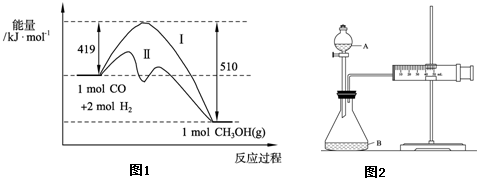


 (mol��L-1��min-1)
(mol��L-1��min-1) ��С
��С