��Ŀ����
A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ��������֮�������µķ�Ӧ��ϵ����Ӧ���е�ˮû�������A+B��C+D
��1����������Ӧ�ǹ�ҵ�Ʋ�������Ҫ��Ӧ֮һ��BΪ�������B�Ļ�ѧʽΪ
��2����AΪ�����ڵĽ������ʣ�DΪ��̬���ʣ�B��Һ��ǿ���Ի�ǿ����ʱ���÷�Ӧ���ܽ��У���д����Ӧ�����ӷ���ʽ
��3�������£���AΪ����ɫ���壬A��B����ʹƷ����Һ��ɫ����Է�������C��D����A��B��ˮ��Һ�з�Ӧ��ѧ����ʽΪ
��1����������Ӧ�ǹ�ҵ�Ʋ�������Ҫ��Ӧ֮һ��BΪ�������B�Ļ�ѧʽΪ
SiO2
SiO2
��B���������������ά
���ά
��������ͨѶ���������䡢��Ϣ�����ȣ���2����AΪ�����ڵĽ������ʣ�DΪ��̬���ʣ�B��Һ��ǿ���Ի�ǿ����ʱ���÷�Ӧ���ܽ��У���д����Ӧ�����ӷ���ʽ
2Al+6H+=2Al3++3H2��
2Al+6H+=2Al3++3H2��
��2Al++2H2O+2OH-=2AlO2-+3H2��
2Al++2H2O+2OH-=2AlO2-+3H2��
����3�������£���AΪ����ɫ���壬A��B����ʹƷ����Һ��ɫ����Է�������C��D����A��B��ˮ��Һ�з�Ӧ��ѧ����ʽΪ
Cl2+SO2+2H2O=2HCl+H2SO4
Cl2+SO2+2H2O=2HCl+H2SO4
������D�������ӵ��Լ�����������Һ��ϡ����
��������Һ��ϡ����
����������1����ҵ���ö��������̼���η�Ӧ���������������������������ά��
��2�����ܺ�ǿ�ᷴӦ���ܺ�ǿ�Ӧ�Ľ���������Al�����ݽ���������������д��
��3������ɫ�����������������Ͷ������������ʹƷ����ɫ�����ݶ��ߵ���������д����ʽ�������ӵļ�������������
��2�����ܺ�ǿ�ᷴӦ���ܺ�ǿ�Ӧ�Ľ���������Al�����ݽ���������������д��
��3������ɫ�����������������Ͷ������������ʹƷ����ɫ�����ݶ��ߵ���������д����ʽ�������ӵļ�������������
����⣺��1��������Ӧ�ǹ�ҵ�Ʋ�������Ҫ��Ӧ�����������̼���εķ�Ӧ��BΪ�����TΪ�������裬���������������ά���ʴ�Ϊ��SiO2�����ά��
��2�����ܺ�ǿ�ᷴӦ���ܺ�ǿ�Ӧ�Ľ���������Al��������������������DΪ��������Ӧԭ������ʽΪ��2Al+6H+=2Al3++3H2����2Al++2H2O+2OH-=2AlO2-+3H2����
�ʴ�Ϊ��2Al+6H+=2Al3++3H2����2Al++2H2O+2OH-=2AlO2-+3H2����
��3������ɫ�����������������Ͷ������������ʹƷ����ɫ�����߷�Ӧ��ԭ������ʽΪ��Cl2+SO2+2H2O=2HCl+H2SO4����Է�������C��D������C�����ᣬD�����ᣬ�����ӵļ��飺���������ữ�������������������ɫ������֤�����������ӣ���֮��û�У��ʴ�Ϊ��Cl2+SO2+2H2O=2HCl+H2SO4����������Һ��ϡ���ᣮ
��2�����ܺ�ǿ�ᷴӦ���ܺ�ǿ�Ӧ�Ľ���������Al��������������������DΪ��������Ӧԭ������ʽΪ��2Al+6H+=2Al3++3H2����2Al++2H2O+2OH-=2AlO2-+3H2����
�ʴ�Ϊ��2Al+6H+=2Al3++3H2����2Al++2H2O+2OH-=2AlO2-+3H2����
��3������ɫ�����������������Ͷ������������ʹƷ����ɫ�����߷�Ӧ��ԭ������ʽΪ��Cl2+SO2+2H2O=2HCl+H2SO4����Է�������C��D������C�����ᣬD�����ᣬ�����ӵļ��飺���������ữ�������������������ɫ������֤�����������ӣ���֮��û�У��ʴ�Ϊ��Cl2+SO2+2H2O=2HCl+H2SO4����������Һ��ϡ���ᣮ
������������һ������ͼ�ƶ��⣬����ѧ�������ͽ��������������ۺ��Խ�ǿ���Ѷȴ�

��ϰ��ϵ�д�
�����Ŀ
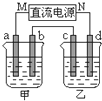 ��ͼ��ʾ��װ���У��ס������ձ��зֱ�ʢ����������CuSO4��Һ��100g 10.00%��K2SO4��Һ��a��b��c��d��Ϊʯī�缫����ͨ��Դһ��ʱ��������K2SO4��ҺŨ��Ϊ10.47%������a�缫���������ӣ�����˵����ȷ���ǣ�������
��ͼ��ʾ��װ���У��ס������ձ��зֱ�ʢ����������CuSO4��Һ��100g 10.00%��K2SO4��Һ��a��b��c��d��Ϊʯī�缫����ͨ��Դһ��ʱ��������K2SO4��ҺŨ��Ϊ10.47%������a�缫���������ӣ�����˵����ȷ���ǣ�������| A���ס�����Һ��pH����С | B���缫b��������������ԼΪ2.8L����״���£� | C���缫d�Ϸ����ķ�ӦΪ��2H2O+2e-?H2��+2OH- | D����ʹ���е���Һ�ָ���ԭ����Ũ�ȣ��ɼ���24.5g��Cu��OH��2 |
 A��B��C��D��Ϊ�������ʣ��֮��Ĺ�ϵ��ͼ��ʾ����-����ʾ�������ʼ��ܷ�����Ӧ����������ʾ���ʼ��ת����ϵ�����ַ�Ӧ����������Լ���Ӧ��������ȥ����
A��B��C��D��Ϊ�������ʣ��֮��Ĺ�ϵ��ͼ��ʾ����-����ʾ�������ʼ��ܷ�����Ӧ����������ʾ���ʼ��ת����ϵ�����ַ�Ӧ����������Լ���Ӧ��������ȥ����
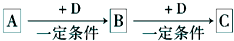 A��B��C��D��Ϊ��ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ�����ֲ�������ȥ�����Իش�
A��B��C��D��Ϊ��ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ�����ֲ�������ȥ�����Իش�
 A��B��C��D��Ϊ������Ԫ�أ�A��B��ͬ�������ڵ�����Ԫ�أ�A��C��ͬ�������ڵ�����Ԫ�أ�A��B��C����Ԫ�ص�ԭ������֮��Ϊ31��DԪ����A��B��C����Ԫ�ؼȲ���ͬ���ڣ�Ҳ��ͬ���壮��ش�
A��B��C��D��Ϊ������Ԫ�أ�A��B��ͬ�������ڵ�����Ԫ�أ�A��C��ͬ�������ڵ�����Ԫ�أ�A��B��C����Ԫ�ص�ԭ������֮��Ϊ31��DԪ����A��B��C����Ԫ�ؼȲ���ͬ���ڣ�Ҳ��ͬ���壮��ش�
 NH3?H2O+H+
NH3?H2O+H+ 2NH3
2NH3
 ���ͱ����γ����ֻ�����X��Y��X��ˮ��Ӧ������һ�־��л�ԭ�ԵĶ�Ԫ��M��1mol�����������ɻ�������Z�����õ�Z����ˮ��Ӧ�IJ���W����12mol KOH������ȫ�кͣ�������������ȼ�����ɻ�����N��N��ˮ��Ӧ����W��DԪ�ص���̬�⻯��Իش��������⣺
���ͱ����γ����ֻ�����X��Y��X��ˮ��Ӧ������һ�־��л�ԭ�ԵĶ�Ԫ��M��1mol�����������ɻ�������Z�����õ�Z����ˮ��Ӧ�IJ���W����12mol KOH������ȫ�кͣ�������������ȼ�����ɻ�����N��N��ˮ��Ӧ����W��DԪ�ص���̬�⻯��Իش��������⣺