��Ŀ����
����Ŀ�������ƻ���к��е��ۡ������Ǻ����εȣ�ij������ȤС�������һ��ʵ��֤��ijЩ�ɷֵĴ��ڣ�������벢Э������������ʵ�顣
��1����С�Թ�ȡ������ƻ��֭������_______(�����ƣ�����Һ��������֤��ƻ���к��е��ۡ�
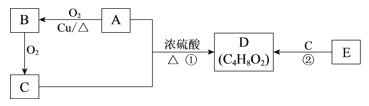
��2�����ú����۵����ʿ����������ᡣ�������������������ͼ���Իش��������⣺
![]()
B���ճ���������������ζ�ij����л������Щ�����к���B��
��д����ѧ����ʽ����ע����Ӧ���͡�
B����ᷴӦ:____________________________������______________��Ӧ��
B��C____________________________������____________________________��Ӧ��
�ڿ����ڼ���A���Լ���____________________________��
���𰸡���ˮ CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O ȡ������������ 2CH3CH2OH+O2
CH3COOCH2CH3+H2O ȡ������������ 2CH3CH2OH+O2![]() 2CH3CHO+2H2O ���� ���Ƶ�������ͭ����Һ�������𰸾��ɣ�
2CH3CHO+2H2O ���� ���Ƶ�������ͭ����Һ�������𰸾��ɣ�
��������
(1)�ⵥ�������۱���ɫ��
(2)������������������ˮ�����������ǣ��������ڴ����������������Ҵ����Ҵ��ɱ�����Ϊ��ȩ���������������
���������к���ȩ����
(1)ƻ���к��е��ۣ��ⵥ�������۱���ɫ����ƻ��֭�м����ˮ����Һ��������ƻ���к��е��ۣ�
(2)����������������ˮ�����������ǣ��������ڴ����������������Ҵ����Ҵ��ɱ�����Ϊ��ȩ���������������ᣬ��AΪ�����ǣ�BΪ�Ҵ���CΪ��ȩ��
���Ҵ���������Ũ���������·�������(ȡ��)��Ӧ����������������Ӧ�ķ���ʽΪCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��BΪ�Ҵ���CΪ��ȩ���Ҵ����ڴ��������·���������������ȩ����Ӧ�ķ���ʽΪ2CH3CH2OH+O2
CH3COOCH2CH3+H2O��BΪ�Ҵ���CΪ��ȩ���Ҵ����ڴ��������·���������������ȩ����Ӧ�ķ���ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
���������к���ȩ�������������ǿ������Ƶ�������ͭ����Һ�����Ⱥ�������ש��ɫ�����������ˮ�����������ǡ�

 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�