��Ŀ����
����Ŀ���±���Ԫ�����ڱ���һ���֣��ش������й����⣺ 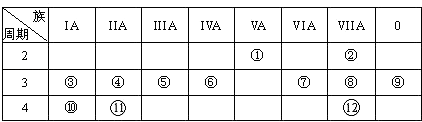
��1��д������Ԫ�ط��ţ��� �� �� �� �� ��
��2������ԭ�ӵĽṹʾ��ͼ���� �� �� ��
��3���ڢ١�12Ԫ���У���������ǿ��Ԫ���� �� �ǽ�������ǿ��Ԫ���� �� ����õ�Ԫ���� �� ������Ԫ�ط��ţ�
��4��Ԫ�آ���Ԫ�آ���ȣ��ǽ����Խ�ǿ��������Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ���� ��
a�������¢ߵĵ��ʺ͢�ĵ���״̬��ͬ
b������⻯��Ȣߵ��⻯���ȶ�
c��һ�������¢ߺ͢�ĵ��ʶ���������������Һ��Ӧ
��5������������ԭ�Ӱ뾶����Ԫ����������ţ�������ͬ����ԭ�Ӱ뾶��С��Ԫ����������ţ������ǿ����γ��������ӻۣ�������õ���ʽ��ʾ���γɹ������£� ��
��6����֪ijԪ��ԭ���������������������������2������Ԫ�ؿ�������γ�һ��AB4�͵Ļ�������õ���ʽ��ʾ���γɹ��̣� ��
���𰸡�
��1��N��Si��S
��2��![]() ��
��![]()
��3��K��F��Ar
��4��Cl��b
��5���ۣ��ࣻ���ӣ�![]()
��6��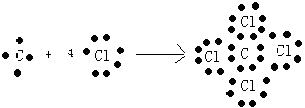
���������⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪN����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪS����ΪCl����ΪAr����ΪK����11��ΪCa����12��ΪBr.
��1.��������������֪��N����Si����S�����Դ��ǣ�N��Si��S��
��2.��Mg��Ԫ�����ڱ��е������ڵڢ�A�壬ԭ�ӽṹʾ��ͼΪ ![]() ��Cl��Ԫ�����ڱ��е������ڵڢ���A�壬ԭ�ӽṹʾ��ͼΪ��
��Cl��Ԫ�����ڱ��е������ڵڢ���A�壬ԭ�ӽṹʾ��ͼΪ�� 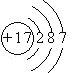 �����Դ��ǣ�
�����Դ��ǣ� ![]() ��
�� 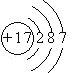
��3.������Ԫ���н�������ǿ����K���ǽ�������ǿ����F��Ar������������Ϊ8����Ar��������Դ��ǣ�K��F��Ar��
��4.����ΪS����ΪCl��ͬ����Ԫ�ش�������Ԫ�صķǽ�����ǿ����Cl�ķǽ�����ǿ����������̬�⻯����ȶ�������֤�����Դ��ǣ�Cl��b��
��5.���������ڴ�������ԭ�Ӱ뾶�ڼ�С����Na��ԭ�Ӱ뾶���ѡ�ۣ�ԭ�Ӱ뾶��С��ΪCl����ѡ�࣬�����γɵĻ�����ΪNaCl��Ϊ���ӻ����
���γɹ���Ϊ ![]() �����Դ��ǣ��ۣ��ࣻ���ӣ�
�����Դ��ǣ��ۣ��ࣻ���ӣ� ![]() ��
��
��6.��ijԪ��ԭ���������������������������2������Ԫ��ΪC������γ�һ��AB4�͵Ļ�����ΪCCl4 �� ���γɹ���Ϊ 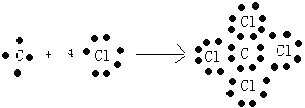 ��
��
���Դ��ǣ� 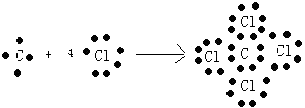 ��
��

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�����Ŀ��ij��ѧ��ȤС��Ҫ����к��ȵIJⶨ��
��1��ʵ�����ϱ����ձ�(��С�����ձ�)����ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β�������0.5mol��L-1���ᡢ0.55mol��L-1NaOH��Һ����ȱ�ٵ�ʵ�鲣����Ʒ��______________��_________________��
ʵ����Ʒ | ��Һ�¶� | �к��ȡ�H | |||
t1 | t2 | ||||
�� | 50mL0.55mol��L-1NaOH | 50mL.0.5mol��L-1HCl | 20�� | 23.3�� | _______ |
�� | 50mL0.55mol��L-1NaOH | 50mL.0.5mol��L-1HCl | 20�� | 23.5�� | |
��2�����Ǽ�¼��ʵ���������£�
��֪��Q=Cm(t2-t1)����Ӧ����Һ�ı�����CΪ4.18kJ����-1��kg��1�������ʵ��ܶȾ�Ϊlg��cm-3����������ϱ�����H=____________
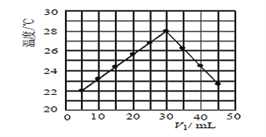
��3��ij�о�С�齫V1mL 1.0 mol/L HCl��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ��������ͼ��ʾ(ʵ����ʼ�ձ���V1+V2 =50mL)���˷�Ӧ����NaOH��Һ��Ũ��ӦΪ__________mol/L��