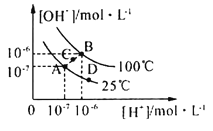
��Ŀ����
����Ŀ��һ��ʵ�����Ʊ��������ƺ�����ص�װ�úͲ������£�
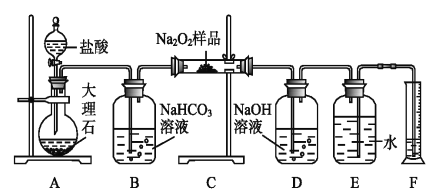
����ͼ��װװ�ã����װ�õ������ԡ�������ƿA�ڷ���5gKMnO4��ĩ����ȫ©�������Թ��ڲ������Թ�5�з���4mL6mol��L-1��KOH��Һ���Թ�6�з���4mL2mol��L-1��NaOH��Һ���۴�ֹˮ��3���ر�ֹˮ��4�������Թ�5���¶���323��328K��Χ�ڣ�ͨ����ȫ©������ƿA�л�������15mL9mol��L-1�����ᣬ�������������Ȳ������ܵ��Թ�5������ɫ������Ϊ����ɫ�����ɻ���ɫͻȻ�����ɫʱ������ͨ��������Һ�ʼ����Ļ�ɫ��ֹͣ���ȡ��ݡ����Թ�5���£���ˮԡ��ȴ�������о������������ˡ�ϴ�ӡ����
�ش��������⣺
(1)��ƿA�ڵ�С�Թܵ�������___��
(2)ϴ��ƿ2�ڵ��Լ�������___��
A.�����Ȼ�����Һ B.Ũ���� C.����ʳ��ˮ D.����̼��������Һ
(3)�����Թ�5���¶���323��328K��Χ�ڣ���ȡ�ļ��ȷ�ʽ��___���Թ�5��������KClO���Ի���ɫ��KClO�ĵ���ʽΪ___������ɫͻȻ��Ϊ��ɫ����ΪKClO�ֽ�������KClO3��д��KClO�ֽ�Ļ�ѧ����ʽ��___��
(4)����ݵIJ�����___��
(5)������еõ��ľ������Ҵ�ϴ�ӵ��ŵ���___��
(6)ȡ�Թ�6�е�Һ����������һ�Թ��У�����������������ԣ������еμ�0.2mol��L-1��MnSO4��Һ���к�ɫ�������ɣ�������Ӧ�����ӷ���ʽΪ___��
���𰸡�Һ�⣬��ֹ���ɵ������ݳ� AC ˮԡ���� ![]() 3KClO
3KClO![]() 2KCl+KClO3 ��ֹˮ��4���ر�ֹˮ��3 ��������ص��ܽ���ʧ�������ڿ��ٸ��� ClO-+Mn2++H2O=MnO2��+Cl-+2H+
2KCl+KClO3 ��ֹˮ��4���ر�ֹˮ��3 ��������ص��ܽ���ʧ�������ڿ��ٸ��� ClO-+Mn2++H2O=MnO2��+Cl-+2H+
��������
�������̺Ͳ��裬��ƿA�ڷ���KMnO4��ĩ�������������ᣬ���������������ص��ܾ���ϴ��ƿ2��ͨ��ֹˮ��3�����Թ�5������Ӧ���Թ�5�з���KOH��Һ����ô��Ʊ�����أ��Թ�6�з���NaOH��Һ���ô��Ʊ��������ƣ�B��������ƿ�е�NaOH��ҺĿ���������չ�������������ֹ��Ⱦ������ϴ��ƿ2��Ŀ�����ڳ�ȥ���������е��Ȼ������塣
(1)��ͼ��֪�����������õ��dz���©��������������ͨ��Ϊ��ֹ�����ݳ����ʷ���һ��С�Թܣ�������ƿA�ڵ�С�Թܵ�������Һ�⣬��ֹ���ɵ������ݳ���
(2)ϴ��ƿ2��Ŀ�����ڳ�ȥ���������е��Ȼ������壬���Լ�һ����Ҫ�����Ȼ��⣬��һ����Ҫ��ֹ�����ܽ⣬����������Ϊ�����Ȼ�����Һ ������ʳ��ˮ����ѡAC��
(3)�Թ�5���¶���323��328K��Χ�ڣ���50��-55�棬�¶ȷ�ΧС����ˮ�ķе㷶Χ�ڣ���ȡ�ļ��ȷ�ʽ��ˮԡ���ȣ�KClO�д������Ӽ����ۼ�������ʽΪ![]() ��KClO�ֽ�������KClO3���ȵĻ��ϼۼ������ֽ��ͣ����ݵ�ʧ�����غ���ƽ����KClO�ֽ�Ļ�ѧ����ʽΪ3KClO
��KClO�ֽ�������KClO3���ȵĻ��ϼۼ������ֽ��ͣ����ݵ�ʧ�����غ���ƽ����KClO�ֽ�Ļ�ѧ����ʽΪ3KClO![]() 2KCl+KClO3��
2KCl+KClO3��
(4)����ܷ�Ӧ�Ѿ���ɣ��ʲ���ݵIJ����Ǵ�ֹˮ��4���ر�ֹˮ��3��
(5)�Ҵ����лӷ��ԣ����Ҵ�ϴ�ӿ��Կ��ٸ���������ˮϴ�ӣ�����ص��ܽ���ʧҪС�������Ҵ�ϴ�ӵ��ŵ��Ǽ�������ص��ܽ���ʧ�������ڿ��ٸ��
(5)�Թ�6�Ʊ��������ƣ��������������������¾���ǿ�����ԣ������еμ�MnSO4��Һ���к�ɫ�������ɣ��ó���ӦΪMnO2��������Ӧ�����ӷ���ʽΪ
ClO-+Mn2++H2O=MnO2��+Cl-+2H+��

 �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�