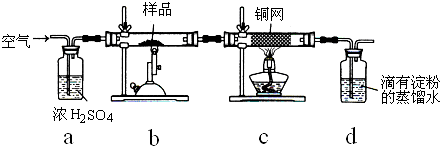
��Ŀ����
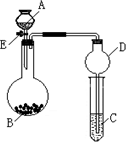 ijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飬ʵ��ʱ��ҩƷA��μ��뵽����B�У����������ʵ��ش����⣺
ijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飬ʵ��ʱ��ҩƷA��μ��뵽����B�У����������ʵ��ش����⣺��1����AΪˮ��BΪ�������ƣ�C��ʢ�������ữ����FeCl2��Һ����������E��B�з�����Ӧ�Ļ�ѧ����ʽΪ
2Na2O2+2H2O=4NaOH+O2
2Na2O2+2H2O=4NaOH+O2
��C������Ϊ��ƻ����
��ƻ����
����2����AΪ���ᣬBΪ�������ƹ��壬C��ʢ����������Һ����������E��B�з�����Ӧ�Ļ�ѧ����ʽ��Ϊ
H2SO4+Na2SO3=Na2SO4+H2O+SO2
H2SO4+Na2SO3=Na2SO4+H2O+SO2
��C�з�����Ӧ�Ļ�ѧ����ʽ��Ϊ2H2S+SO2=3S+2H2O
2H2S+SO2=3S+2H2O
����3����������װ�û�������֤���ʵ����ʣ������֤�������ԣ�KClO3��Cl2��Br2����A�м�Ũ���ᣬB�м�
KClO3
KClO3
��C�з�����Ӧ�����ӷ���ʽΪ2Br-+Cl2=2Cl-+Br2
2Br-+Cl2=2Cl-+Br2
����������1������������ˮ��Ӧ�����������������Ӿ��л�ԭ�ԣ���Һ�����dz��ɫ�������������������ӣ���Һ��ƣ�
��2���������������Ʒ�Ӧ���ɶ����������壬����������������ԣ�������л�ԭ�ԣ����߷���������ԭ��Ӧ���ɵ�����
��3������������+��ԭ��=��������+��ԭ��������ԣ������������������ԭ�ԣ���ԭ������ԭ����жϿ��ܷ����ķ�Ӧ��
��2���������������Ʒ�Ӧ���ɶ����������壬����������������ԣ�������л�ԭ�ԣ����߷���������ԭ��Ӧ���ɵ�����
��3������������+��ԭ��=��������+��ԭ��������ԣ������������������ԭ�ԣ���ԭ������ԭ����жϿ��ܷ����ķ�Ӧ��
����⣺��1������������ˮ��Ӧ������������Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2H2O�T4NaOH+O2����������ǿ�����ԣ��ܽ�Fe2+����������Fe3+���ӣ���Һ��ƣ�
�ʴ�Ϊ��2Na2O2+2H2O�T4NaOH+O2����ƻ���
��2���������������Ʒ�Ӧ���ɶ����������壬��Ӧ����ʽΪH2SO4+Na2SO3�TNa2SO4+H2O+SO2������������������ԣ�������л�ԭ�ԣ����߷���������ԭ��Ӧ���ɵ�����Ӧ�Ļ�ѧ����ʽΪ2H2S+SO2�T3S+2H2O���ʴ�Ϊ��H2SO4+Na2SO3�TNa2SO4+H2O+SO2��2H2S+SO2�T3S+2H2O��
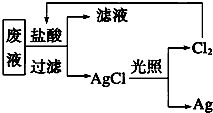
��3������������+��ԭ��=��������+��ԭ��������ԣ������������������ԭ�ԣ���ԭ������ԭ���֤�������ԣ�KClO3��Cl2��Br2���ɽ�KClO3��Ϊ�����������ᷴӦ�ж��Ƿ����������������������Դ���Br2��������ͨ��NaBr��Һ����Һ��Ϊ�Ⱥ�ɫ��˵����Br2���ɣ���Ӧ�Ļ�ѧ����ʽΪ2Br-+Cl2�T2Cl-+Br2��
�ʴ�Ϊ��KClO3��2Br-+Cl2�T2Cl-+Br2��
�ʴ�Ϊ��2Na2O2+2H2O�T4NaOH+O2����ƻ���
��2���������������Ʒ�Ӧ���ɶ����������壬��Ӧ����ʽΪH2SO4+Na2SO3�TNa2SO4+H2O+SO2������������������ԣ�������л�ԭ�ԣ����߷���������ԭ��Ӧ���ɵ�����Ӧ�Ļ�ѧ����ʽΪ2H2S+SO2�T3S+2H2O���ʴ�Ϊ��H2SO4+Na2SO3�TNa2SO4+H2O+SO2��2H2S+SO2�T3S+2H2O��
��3������������+��ԭ��=��������+��ԭ��������ԣ������������������ԭ�ԣ���ԭ������ԭ���֤�������ԣ�KClO3��Cl2��Br2���ɽ�KClO3��Ϊ�����������ᷴӦ�ж��Ƿ����������������������Դ���Br2��������ͨ��NaBr��Һ����Һ��Ϊ�Ⱥ�ɫ��˵����Br2���ɣ���Ӧ�Ļ�ѧ����ʽΪ2Br-+Cl2�T2Cl-+Br2��
�ʴ�Ϊ��KClO3��2Br-+Cl2�T2Cl-+Br2��
���������⿼��Ԫ�ػ�����֪ʶ����Ŀ�ѶȲ���ע��������ơ��������Ƶ����ʣ��Լ�ʵ����Ƶķ�������ƣ�

��ϰ��ϵ�д�
�����Ŀ
 ����Ժǿ��������ˮ���������̱����ֽ�ˮ�����ۺ���̬���������빤�̽�����Э������ˮ��Դ��������Ϊ���ߣ��ѽ�ˮ�����ۡ���̬�����������ˮ��Ϊһ��������ϵͳ��������ԭ���ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ�������������ij������Һ�У����д�����Mg2+��Al3+��Cu2+��Ag+���Է����ش��������⣺
����Ժǿ��������ˮ���������̱����ֽ�ˮ�����ۺ���̬���������빤�̽�����Э������ˮ��Դ��������Ϊ���ߣ��ѽ�ˮ�����ۡ���̬�����������ˮ��Ϊһ��������ϵͳ��������ԭ���ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ�������������ij������Һ�У����д�����Mg2+��Al3+��Cu2+��Ag+���Է����ش��������⣺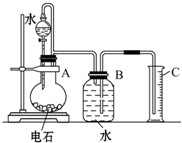 ijͬѧ�������ͼ��ʾ��ʵ��װ�������Եزⶨ��ʯ��̼���Ƶ�����������
ijͬѧ�������ͼ��ʾ��ʵ��װ�������Եزⶨ��ʯ��̼���Ƶ�����������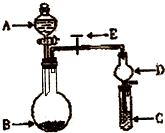 ��2010?����һģ����ѧʵ����һ��װ������������ɶ��ʵ�飬Aijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飮��ش�
��2010?����һģ����ѧʵ����һ��װ������������ɶ��ʵ�飬Aijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飮��ش�