��Ŀ����
 ����Ժǿ��������ˮ���������̱����ֽ�ˮ�����ۺ���̬���������빤�̽�����Э������ˮ��Դ��������Ϊ���ߣ��ѽ�ˮ�����ۡ���̬�����������ˮ��Ϊһ��������ϵͳ��������ԭ���ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ�������������ij������Һ�У����д�����Mg2+��Al3+��Cu2+��Ag+���Է����ش��������⣺
����Ժǿ��������ˮ���������̱����ֽ�ˮ�����ۺ���̬���������빤�̽�����Э������ˮ��Դ��������Ϊ���ߣ��ѽ�ˮ�����ۡ���̬�����������ˮ��Ϊһ��������ϵͳ��������ԭ���ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ�������������ij������Һ�У����д�����Mg2+��Al3+��Cu2+��Ag+���Է����ش��������⣺��1���÷�Һ�п��ܴ������ڵ�һ����������
D
D
��ѡ����ţ�?A��SO42- B��CO32- C��Cl- D��NO3-
��2��������Һ����Ԫ�صĺ����ϸߣ��轫���������������ӷֿ�����ѡ�ú��ʵ��Լ���д����Ԫ������Լ���Ӧʱ�����ӷ���ʽ��
Al3++4OH-=AlO2-+2H2O
Al3++4OH-=AlO2-+2H2O
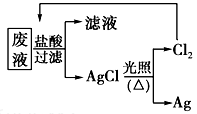
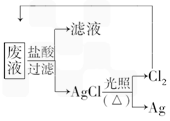
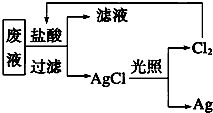
����3��Ϊ�˻��շ�Һ�еĽ�������ijͬѧ�������ͼ��ʾ�ķ����������÷�����ý�����10.80g��Ϊ��֤����Ⱦ������Cl2��ѭ�����ã�������Ӧ�ṩ��״���µ�H2���Ϊ
1.12
1.12
L����������1����Mg2+��Al3+��Cu2+��Ag+���κ����Ӷ�����Ӧ�����ӿɴ������棻
��2��������������Ϊ����������������ʽ��з��룻
��3��n��Ag��=
=0.1mol����Ϸ�Ӧ�Ĺ�ϵʽ2AgCl��Cl2��H2���㣮
��2��������������Ϊ����������������ʽ��з��룻
��3��n��Ag��=
| 10.80g |
| 108g/mol |
����⣺��1������SO42-��CO32-��Cl-������Ag+��Ӧ����������ˮ������ˮ�ij�����ֻ��D���ϣ��ʴ�Ϊ��D��
��2����������Ϊ���������������ǿ����룬��ط�Ӧ�����ӷ���ʽΪAl3++4OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al3++4OH-=AlO2-+2H2O��
��3��n��Ag��=
=0.1mol����Ӧ�Ĺ�ϵʽΪ2AgCl��Cl2��H2����n��H2��=0.05mol��
V��H2��=0.05mol��22.4L/mol=1.12L��
�ʴ�Ϊ��1.12��
��2����������Ϊ���������������ǿ����룬��ط�Ӧ�����ӷ���ʽΪAl3++4OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al3++4OH-=AlO2-+2H2O��
��3��n��Ag��=
| 10.80g |
| 108g/mol |
V��H2��=0.05mol��22.4L/mol=1.12L��
�ʴ�Ϊ��1.12��
���������⿼���Ϊ�ۺϣ��漰���ӹ��桢���ӷ���ʽ����д�����㣬Ϊ�߿��������ͣ�������ѧ��Ԫ�ػ�����֪ʶ���ۺ����ã��ѶȲ���ע����������Ϣ�Լ�������ӵ����ʣ�

��ϰ��ϵ�д�
 �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ
| |||||||||||||||||||||||||