��Ŀ����
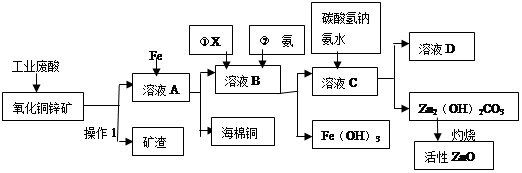
ij����С����Ƴ����ù�ҵ���ᣨ10%H2SO4�����ѽ�ij����������ͭп����Ҫ�ɷݣ�CuO ZnO���ķ�����ʵ�ַ����ۺ����ã���������ͼ��ʾ��
��ش��������⣺
��1������1�������ǣ�
��2������ҺA�м���Fe�۷�������Ҫ�ķ�Ӧ���ӷ���ʽΪ�� �� ��
��3������ҺB�м�������X��Ŀ���� ������X�������������е� ��(����ĸ)
��4������ҺB�м��백ˮ��Ŀ���ǵ�����Һ��pH��pHӦ������ ��Χ֮�䡣
��5����ҺD����Ҫ���ʵĻ�ѧʽ�� ��
��6������ͭп���к���������CuS��ZnS����H2SO4��������ZnS�����ܽ��CuS���ܣ�����ͬ�¶��£�Ksp(CuS) Ksp(ZnS)��ѡ�������������������

��ش��������⣺
��1������1�������ǣ�
��2������ҺA�м���Fe�۷�������Ҫ�ķ�Ӧ���ӷ���ʽΪ�� �� ��
��3������ҺB�м�������X��Ŀ���� ������X�������������е� ��(����ĸ)
| A��KMnO4 | B��O2 | C��H2O2 | D��NaOH |
��5����ҺD����Ҫ���ʵĻ�ѧʽ�� ��
��6������ͭп���к���������CuS��ZnS����H2SO4��������ZnS�����ܽ��CuS���ܣ�����ͬ�¶��£�Ksp(CuS) Ksp(ZnS)��ѡ�������������������
��1�����ˣ�2�֣�
��2��Fe + 2H+��Fe2+ + H2����2�� Fe + Cu2+��Fe2+ + Cu��2�֣�
��3����Fe2+������Fe3+���Ա��ȥ ��2�֣�B C ��2�֣�
��4��3.2 ��PH�� 6.2��2�֣�
��5��(NH4)2SO4 ��2�֣� ��6���� ��2�֣�
��2��Fe + 2H+��Fe2+ + H2����2�� Fe + Cu2+��Fe2+ + Cu��2�֣�
��3����Fe2+������Fe3+���Ա��ȥ ��2�֣�B C ��2�֣�
��4��3.2 ��PH�� 6.2��2�֣�
��5��(NH4)2SO4 ��2�֣� ��6���� ��2�֣�
�����������1������1Ϊ��Һ�����������������Ϊ���ˡ�
��2������ͭп�����������������ܽ�����Cu2+��Zn2+��ͬʱΪ��ʹ�������ܽ⣬�������Ҫ������������������ҺΪ������Һ���ټ���Fe�ۣ�������ӦFe + 2H+��Fe2+ + H2���� Fe + Cu2+��Fe2+ + Cu��
��3����Ϊ�õ���B��ҺҪ��������Fe��OH��3 ��������Ѷ���������Ϊ�������Ա���õij�ȥ�����Գ��˼Ӽ���ˮ��Һ�⣬��Ӧ�ü��������������Ҳ��������ʣ����Կ���ѡ��O2��H2O2����ѡBC��
��4�����백ˮ��Ҫ��ʹFe3+��ȫ����������ʹZn2+����������Ӧ�ÿ���PH��Χ��3.2 ��PH�� 6.2֮��
��5�����ڹ��������μ��백ˮ����C��Һ�е�Zn2+��̼������������˳�����ʽ̼��п������ʣ��D��Һ�к��еĴ���������ΪNH4+������������ΪSO42-��������Ҫ����Ϊ(NH4)2SO4 ��
��6����H2SO4��������ZnS�����ܽ��CuS���ܣ�˵��CuS�����ܣ�������ͬ�¶��£�Ksp(CuS)<Ksp(ZnS)��

��ϰ��ϵ�д�
�����Ŀ

 Cu AlO2 �� ��ϵ��1ҲҪд��.
Cu AlO2 �� ��ϵ��1ҲҪд��.