��Ŀ����
����Ŀ��ͭ�Ļ�������;�dz��㷺����֪���з�Ӧ��[Cu(NH3)2]++NH3+CO [Cu(NH3)3CO]+��2CH3COOH +2CH2��CH2+O2![]() 2CH3COOCH��CH2+2H2O��
2CH3COOCH��CH2+2H2O��
(1)Cu2+��̬��������Ų�ʽΪ______��
(2)NH3���ӿռ乹��Ϊ_______�� ������ԭ�ӵ��ӻ�������______��
(3)CH3COOCH��CH2������̼ԭ�ӹ�����ӻ�������_______��1mol CH3COOCH��CH2�к�![]() ����ĿΪ_____��
����ĿΪ_____��
(4)CH3COOH����H2O���ܣ�����Ϊ���Ƕ��Ǽ��Է����⣬����Ϊ__________��
(5)������[Cu(NH3)3CO]+��NH3��CO�е�C��Cu(��)�γ���λ���������ǿռ乹�ͣ�[Cu(NH3)3CO]+�Ľṹʾ��ͼ��ʾΪ____
���𰸡�[Ar]3d9 ��1s22s22p63s23p63d9 ���� sp3 sp2��sp3 11mol CH3COOH��H2O֮������γ���� 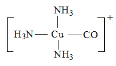
��������
����ͭԭ�ӵĵ����Ų�ʽ��ʧȥ������������һ�����ӣ�����д��ͭ���ӵĵ����Ų�ʽ������VSEPR�ж���ռ乹�ͺ��ӻ������ʽ�����ݽṹ�����������ж��ӻ������ʽ�ͼ������������ݷ��Ӽ�����γ�������ж������ԣ������γ���λ�����жϽṹʾ��ͼ��
��1��CuԪ�صĺ˵����Ϊ29�����������ҲΪ29����̬Cuԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p63d104s1��4s��3d�ܼ���ʧȥ1�������γ�Cu2+��Cu2+��̬��������Ų�ʽΪ1s22s22p63s23p63d9����Ϊ1s22s22p63s23p63d9��
��2��NH3������Nԭ�Ӽ۲���Ӷ���=3+ ![]() =4���Һ���1�Թµ��Ӷԣ�����VSEPRģ��Ϊ�������Σ�����һ�Թµ��Ӷ�ռ���������һ�����㣬������ռ乹��Ϊ�����Σ������ӻ�������ۣ�����Nԭ�ӵ��ӻ���ʽΪsp3�ӻ�����Ϊ�����Σ�sp3��
=4���Һ���1�Թµ��Ӷԣ�����VSEPRģ��Ϊ�������Σ�����һ�Թµ��Ӷ�ռ���������һ�����㣬������ռ乹��Ϊ�����Σ������ӻ�������ۣ�����Nԭ�ӵ��ӻ���ʽΪsp3�ӻ�����Ϊ�����Σ�sp3��
��3��CH3COOCH=CH2�����м��ϵ�C�γ�4��������û�й¶Ե��ӣ�Cԭ��Ϊsp3�ӻ����Ȼ��ϵ�̼��̼̼˫����̼���γ�3��������û�й¶Ե��ӣ�Ϊsp2�ӻ���CH3COOCH=CH2�����к���1��C-C��6��C-H��2��C-O��1��C=O��1��C=C��������������ĿΪ1+6+2+1+1=11��1mol CH3COOCH=CH2�����к�����������ĿΪ11mol����Ϊ��sp2��sp3��11mol��
��4�������ˮ��Ϊ���Է��ӣ��ҷ���֮����γ��������CH3COOH����H2O���ܣ���ΪCH3COOH��H2O֮������γ������
��5��Cu��I���ṩ�չ����N��Cԭ���ṩ�¶Ե��ӣ�Cu��I����NH3��CO�е�C�γ���λ�����ṹʾ��ͼ��ʾΪ��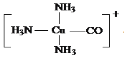 ����Ϊ
����Ϊ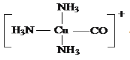 ��
��

����Ŀ�����̿����Ҫ�ɷ���MnCO3������������Fe��Al��Ca��Mg��Ԫ�ء��Ȼ�隣������̿��Ʊ��ߴ���̼���̵Ĺ����������£�

��֪������ؽ������ӣ�c0(Mn+)=0.1 mol/L���γ��������������pH��Χ���£�
�������� | Al3+ | Fe3+ | Fe2+ | Ca2+ | Mn2+ | Mg2+ |
��ʼ������pH | 3.8 | 1.5 | 6.3 | 10.6 | 8.8 | 9.6 |
������ȫ��pH | 5.2 | 2.8 | 8.3 | 12.6 | 10.8 | 11.6 |
�ڳ����£�CaF2��MgF2���ܶȻ������ֱ�Ϊ1.46��10��10����7.42��10��11
�ش��������⣺
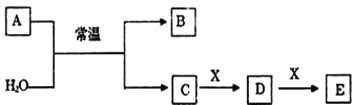
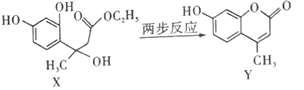
(1) ��������ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ__________������XΪ________���ѧʽ����д���������е�һ����;_______________��
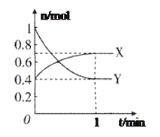
(2)����ʱ�¶ȶ��̽����ʵ�Ӱ����ͼ��ʾ������ʱ��Ӧ�¶�Ϊ_______________��

(3)����Һ�������������������£�������MnO2��Fe2+����ΪFe3+���ٵ�����Һ��pH��Al3+��Fe3+��ɳ�����ȥ����Һ��pH�ķ�ΧΪ____Ȼ�����NH4F��Ca2+��Mg2+ת��ΪCaF2��MgF2������ȥ�����ֳ�������ʱ����Һ��![]() =________�����ý��������λС������
=________�����ý��������λС������
(4)̼���ᾧ��ԭ��(�����ӷ���ʽ��ʾ)�� ______________________��
(5)���Ƶõĸߴ���̼�������ڹ���ϡ������ö��Ե缫��⣬��ij���õ���Ҫ�������ܲ���MnO2���õ缫�ĵ缫��ӦʽΪ_________��
(6)�ڸù��������п���ѭ��ʹ�õ�������__________��(�ѧʽ)
����Ŀ������������ζ����֮�ơ�������ʯ����Ҫ����SrSO4������CaCO3��MgO���ʣ������������ȵĹ������£�

��֪����������ˮ�е��ܽ�ȣ�
�¶ȣ��棩 | 0 | 10 | 20 | 30 | 40 | 60 | 80 | 90 | 100 |
�ܽ�ȣ�g/100mL�� | 0.91 | 1.25 | 1.77 | 2.64 | 3.95 | 8.42 | 20.2 | 44.5 | 91.2 |
��1��������������ʱSrSO4ֻ����ԭ��SrS����ѧ����ʽΪ____��
��2��������������������Һ������95������NaOH��Һ����pHΪ12��
��95��ʱˮ�����ӻ�KW��1.0��10��12��Ksp[Mg(OH)2]��1.2��10��10������Һ��c��Mg2������____��
����pH���������������ȵIJ��ʽ��ͣ������ԭ��____��
��3�������ȹ�������Ŀ����____��������������Ҫ�ɷ�Ϊ___��
��4���ӳ��ȹ��˺����Һ�еõ�Sr(OH)2��Ʒ�IJ���Ϊ____�����ˡ�ϴ�ӡ����
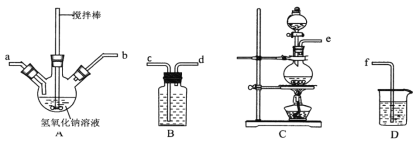
��5������������������FeCl3��Һ����������������壬����ʱ����������Ϊ___���ѧʽ��������ʯī�缫�������Һ�������������������ѭ�����õ�������__��