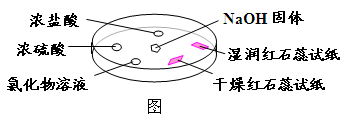
��Ŀ����
��15�֣�����������һ�ֹ�ҵ�Σ�������ͼ��ʾ�������г�װ����ʡ�ԣ���ҩƷ��̽���������������ᷴӦ���������ɷ֡�
��֪����NO��NO2��2OH����2NO2����H2O
������Һ�����¶ȣ�NO2 21�桢NO -152��
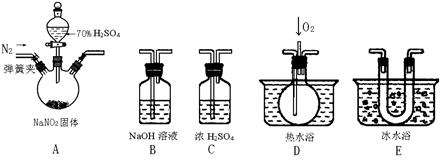
��1��Ϊ�˼���װ��A�����ɵ�����������������˳����������ӣ���
A ��C�� �� �� ��
��2����ӦǰӦ���ɼУ���ͨ��һ��ʱ�䵪�����ų�װ���еĿ�����Ŀ���ǣ�
��
��3���ڹرյ��ɼУ���Һ©������������70%�����A�в�������ɫ���塣
��ȷ��A�в��������庬��NO�����ݵ������� ��
��װ��E�������� ��
��4�������D��ͨ�����O2����װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ �����û��װ��C����ʵ�������ɵ�Ӱ���� ��
��5��ͨ������ʵ��̽�����̣��ɵó�װ��A�з�Ӧ�Ļ�ѧ����ʽ�� ��
��֪����NO��NO2��2OH����2NO2����H2O
������Һ�����¶ȣ�NO2 21�桢NO -152��

��1��Ϊ�˼���װ��A�����ɵ�����������������˳����������ӣ���
A ��C�� �� �� ��
��2����ӦǰӦ���ɼУ���ͨ��һ��ʱ�䵪�����ų�װ���еĿ�����Ŀ���ǣ�
��
��3���ڹرյ��ɼУ���Һ©������������70%�����A�в�������ɫ���塣
��ȷ��A�в��������庬��NO�����ݵ������� ��
��װ��E�������� ��
��4�������D��ͨ�����O2����װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ �����û��װ��C����ʵ�������ɵ�Ӱ���� ��
��5��ͨ������ʵ��̽�����̣��ɵó�װ��A�з�Ӧ�Ļ�ѧ����ʽ�� ��
��1��E��D��B ��3�֣�
��2����ֹ�������ɵ�NO����ȫ������NO2����ɶ�A�з�Ӧ���������鲻��ȫ��2�֣�
��3����D�г��ֺ���ɫ���壨2�֣�������ʹNO2��ȫҺ����2�֣�
��4��4NO2 + O2 + 4NaOH = 4NaNO3 +2 H2O��2�֣�
ˮ�������ڣ�����NO2��Ӧ����NO����ɶ�NO��Դ���������ʶ���壨2�֣�
��5��2NaNO2 + H2SO4 = Na2SO4 + NO2�� + NO��+ H2O��2�֣�
��2����ֹ�������ɵ�NO����ȫ������NO2����ɶ�A�з�Ӧ���������鲻��ȫ��2�֣�
��3����D�г��ֺ���ɫ���壨2�֣�������ʹNO2��ȫҺ����2�֣�
��4��4NO2 + O2 + 4NaOH = 4NaNO3 +2 H2O��2�֣�
ˮ�������ڣ�����NO2��Ӧ����NO����ɶ�NO��Դ���������ʶ���壨2�֣�
��5��2NaNO2 + H2SO4 = Na2SO4 + NO2�� + NO��+ H2O��2�֣�
��1������NO2��Һ���¶ȸߣ��������ȼ���NO2������ͨ��E��Ȼ����ͨ��D�������Ҫ��β��������������EDB��
��2�������е�����������NO�����Ա������ž���������ֹ�������ɵ�NO����ȫ������NO2����ɶ�A�з�Ӧ���������鲻��ȫ��
��3���������NO����װ��E����ȴ����D�е����壬�ܺ�������Ӧ�����ɺ���ɫ���壬��������D�г��ֺ���ɫ���塣
��װ��E������������ʹNO2��ȫҺ����
��4�����������������Bװ�õ�����ȫ����NO2�����Է�Ӧ�ķ���ʽΪ4NO2 + O2 + 4NaOH = 4NaNO3 +2 H2O��������������壬��ˮ�������ڣ�����NO2��Ӧ����NO����ɶ�NO��Դ���������ʶ���塣
��5�����������֪���������������ᷴӦ���������NO2������NO�����Է���ʽΪ2NaNO2 + H2SO4 = Na2SO4 + NO2�� + NO��+ H2O��
��2�������е�����������NO�����Ա������ž���������ֹ�������ɵ�NO����ȫ������NO2����ɶ�A�з�Ӧ���������鲻��ȫ��
��3���������NO����װ��E����ȴ����D�е����壬�ܺ�������Ӧ�����ɺ���ɫ���壬��������D�г��ֺ���ɫ���塣
��װ��E������������ʹNO2��ȫҺ����
��4�����������������Bװ�õ�����ȫ����NO2�����Է�Ӧ�ķ���ʽΪ4NO2 + O2 + 4NaOH = 4NaNO3 +2 H2O��������������壬��ˮ�������ڣ�����NO2��Ӧ����NO����ɶ�NO��Դ���������ʶ���塣
��5�����������֪���������������ᷴӦ���������NO2������NO�����Է���ʽΪ2NaNO2 + H2SO4 = Na2SO4 + NO2�� + NO��+ H2O��

��ϰ��ϵ�д�
�����Ŀ