��Ŀ����
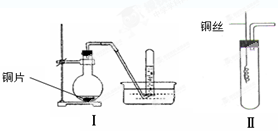
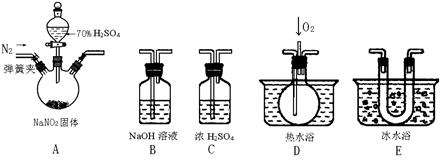
����ͼ������������̽�����������ʡ�ʵ��ʱ��NaOH�����ϵμ���Ũ��ˮ���˷��ɿ����Ʊ�����������������һ������������档�±��ж�ʵ�����������Ľ�����ȷ����
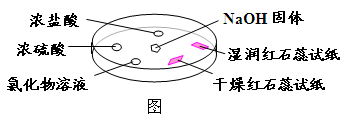

| ѡ�� | ʵ������ | ���� |
| A | Ũ���ḽ���������� | NH3��Ũ���ᷴӦ������NH4Cl���� |
| B | Ũ���ḽ������������ | NH3��Ũ���������Ӧ |
| C | �Ȼ�����Һ����� | ����Һһ����AlCl3��Һ |
| D | �����ʯ����ֽ����ɫ��ʪ���ɫʯ����ֽ���� | NH3��һ�ֿ����Լ� |
A
B����������հ���������Σ�C���Ȼ�þҲ���백�����ɻ��ǣ�D��NH3�����ڼ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ