��Ŀ����
����֪������ˮ�ķ�ӦCl2��H2O![]() HCl��HClO��һ�����淴Ӧ�������ܽ��е��ķ�Ӧ���ҷ�Ӧ���ɵĴ�����(HClO)��һ�����Ա�̼�ỹҪ�����ᡣ�ֱ�д�����ᡢ������ĵ��뷽��ʽ��_______________________��___________________________��
HCl��HClO��һ�����淴Ӧ�������ܽ��е��ķ�Ӧ���ҷ�Ӧ���ɵĴ�����(HClO)��һ�����Ա�̼�ỹҪ�����ᡣ�ֱ�д�����ᡢ������ĵ��뷽��ʽ��_______________________��___________________________��
��þ���Ż�ʱ��������Һ̬CO2����������ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
�ǽ���������Ͷ�뵽����ϡ�����з�����Ӧ�����ӷ���ʽ�� ���ڴ˷�Ӧ�У����������� �Ժ� �ԡ�ʵ��������ȡ���Ļ�ѧ����ʽΪ �� ���ռ���
(1)HCl===H����Cl����HClO![]() H����ClO��
H����ClO��
��2���ԣ���3���ԣ������ԣ����ԣ������ſ�����

��ϰ��ϵ�д�
 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ
 HCl��HClO��һ�����淴Ӧ�������ܽ��е��ķ�Ӧ���ҷ�Ӧ���ɵĴ�����(HClO)��һ�����Ա�̼�ỹҪ�����ᡣд������ĵ��뷽��ʽ��____________________��
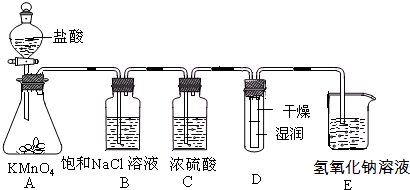
HCl��HClO��һ�����淴Ӧ�������ܽ��е��ķ�Ӧ���ҷ�Ӧ���ɵĴ�����(HClO)��һ�����Ա�̼�ỹҪ�����ᡣд������ĵ��뷽��ʽ��____________________�� ʵ������������ʵ��װ��̽��Cl2���ʲ�ģ���Ʊ�Ưˮ��
ʵ������������ʵ��װ��̽��Cl2���ʲ�ģ���Ʊ�Ưˮ��
 __��
__�� ���µġ����жϸý����Ƿ��������������������ʵ�������ԭ��_________________
���µġ����жϸý����Ƿ��������������������ʵ�������ԭ��_________________ HCl��HClO��һ�����淴Ӧ�������ܽ��е��ķ�Ӧ���ҷ�Ӧ���ɵĴ�����(HClO)��һ�����Ա�̼�ỹҪ�����ᡣд������ĵ��뷽��ʽ��____________________��
HCl��HClO��һ�����淴Ӧ�������ܽ��е��ķ�Ӧ���ҷ�Ӧ���ɵĴ�����(HClO)��һ�����Ա�̼�ỹҪ�����ᡣд������ĵ��뷽��ʽ��____________________��