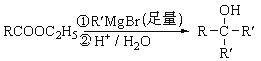��Ŀ����
����Ŀ���¿���M���Ʊ���Ч��������ճ�ϼ��ȶ��־�ϸ��ѧƷ����Ҫԭ�ϣ��ɾ����з�Ӧ·�ߵõ�(���ַ�Ӧ��������)��

��֪����R-CH2-Cl + NaCN �� R-CH2-CN + NaCl
��R-CH2-CN + NaOH + H2O �� R-CH2-COONa + NH3
���������գ�
(1)A�Ľṹ��ʽ��_____________��M�й����ŵ�������________________��
(2)д��B�� C�Ļ�ѧ��Ӧ����ʽ________________________________________��д��G�Ľṹ��ʽ______________________________________
(3)��Ӧa�뷴Ӧb���Ⱥ�˳���ܵߵ�������ԭ��_________________________________________________________________________��
(4)д����A��1,3������ϩ1:1�������ø߾���Ľṹ��ʽ____________________________
(5)�������CH2=CHCH2OH �Ʊ�CH2=CHCOOH�ĺϳ�·��(���Լ���ѡ)________________________________________��
���𰸡�![]() ̼̼˫�����Ȼ�
̼̼˫�����Ȼ� 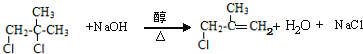
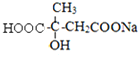 �������ȥ���������������ǻ�ʱ̼̼˫��Ҳ���ܱ�����
�������ȥ���������������ǻ�ʱ̼̼˫��Ҳ���ܱ����� 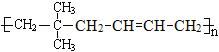 ��
��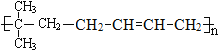 �� CH2=CHCH2OH
�� CH2=CHCH2OH![]() CH3-CHClCH2OH
CH3-CHClCH2OH![]() CH3-CHClCOOH
CH3-CHClCOOH![]() CH2=CHCOONa
CH2=CHCOONa![]() CH2=CHCOOH
CH2=CHCOOH
��������
C��NaCN������Ϣ(1)�ķ�Ӧ����D������D�Ľṹ��֪��CΪ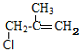 ����B��C�ķ���ʽ��֪��B��ȥ1����HCl������ȥ��Ӧ����C�����A�ķ���ʽ��֪��A����C=C˫���������������ӳɷ�Ӧ����B����BΪ
����B��C�ķ���ʽ��֪��B��ȥ1����HCl������ȥ��Ӧ����C�����A�ķ���ʽ��֪��A����C=C˫���������������ӳɷ�Ӧ����B����BΪ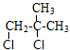 ��AΪ
��AΪ![]() ����F�Ľṹ��֪��D�����������ӳɷ�Ӧ����E��E������Ϣ(2)�е�ˮ�ⷴӦ�õ�F����EΪ
����F�Ľṹ��֪��D�����������ӳɷ�Ӧ����E��E������Ϣ(2)�е�ˮ�ⷴӦ�õ�F����EΪ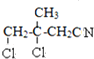 ��Ϊ��ֹ�ǻ�����ʱ��C=C˫������������F�ȷ���������Ӧ����G��G������ȥ��Ӧ���ữ��õ�M����GΪ
��Ϊ��ֹ�ǻ�����ʱ��C=C˫������������F�ȷ���������Ӧ����G��G������ȥ��Ӧ���ữ��õ�M����GΪ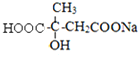 ���ݴ˷������
���ݴ˷������
(1)������������֪��AΪ![]() ������M(
������M(![]() )�Ľṹ��֪�����й�����Ϊ��̼̼˫�����Ȼ����ʴ�Ϊ��
)�Ľṹ��֪�����й�����Ϊ��̼̼˫�����Ȼ����ʴ�Ϊ��![]() ��̼̼˫�����Ȼ���
��̼̼˫�����Ȼ���
(2)B��C�Ļ�ѧ����ʽΪ�� ��G�Ľṹ��ʽΪ��
��G�Ľṹ��ʽΪ��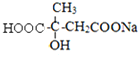 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��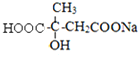 ��
��
(3)�������ȥ���������������ǻ�ʱ̼̼˫��Ҳ���ܱ��������ʷ�Ӧa�뷴Ӧb���Ⱥ�����ܵߵ����ʴ�Ϊ���������ȥ���������������ǻ�ʱ̼̼˫��Ҳ���ܱ�������
(4)��A(![]() )��1��3������ϩ(CH2=CH-CH=CH2)1:1�������ø߾���Ľṹ��ʽΪ
)��1��3������ϩ(CH2=CH-CH=CH2)1:1�������ø߾���Ľṹ��ʽΪ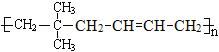 ��
��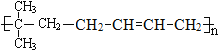 ���ʴ�Ϊ��
���ʴ�Ϊ��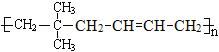 ��
��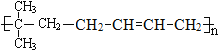 ��
��
(5) ��CH2=CHCH2OH �Ʊ�CH2=CHCOOH����Ҫ�����ǻ����������Ȼ���������ǰ��Ҫ����̼̼˫������˺ϳ�·��ΪCH2=CHCH2OH![]() CH3-CHClCH2OH
CH3-CHClCH2OH![]() CH3-CHClCOOH
CH3-CHClCOOH![]() CH2=CHCOONa
CH2=CHCOONa![]() CH2=CHCOOH���ʴ�Ϊ��CH2=CHCH2OH
CH2=CHCOOH���ʴ�Ϊ��CH2=CHCH2OH![]() CH3-CHClCH2OH
CH3-CHClCH2OH![]() CH3-CHClCOOH
CH3-CHClCOOH![]() CH2=CHCOONa
CH2=CHCOONa![]() CH2=CHCOOH��
CH2=CHCOOH��

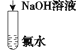
����Ŀ����֪��ϡ��Һ�ǿ���ǿ����кͷ�Ӧ����1 molҺ̬ˮʱ��Ӧ�Ƚ����к��ȡ���������ͼװ�ý����к��ȵIJⶨ����ش��������⣺
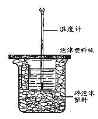
��1��ͼ��δ������ʵ��������______________��
��2���ձ���������ֽ���������� ___________________________��
��3��ʵ��ʱ����0.50 mol��L��1��������뵽0.55mol��L��1��NaOH��Һ�У�������Һ�������Ϊ50 mL������Һ���ܶȾ�Ϊ1 g /cm3��������Һ�ı�����c=4.18 J /(g�� oC)��ʵ�����ʼ�¶�Ϊt1 oC����ֹ�¶�Ϊt2 oC������¶ȱ仯�������£�
ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
���� | NaOH��Һ | ||
1 | 20.2 | 20.3 | 23.7 |
2 | 20.3 | 20.5 | 23.8 |
3 | 21.5 | 21.6 | 24.9 |
���Լ�������ʵ�������к�����H1��______________��
�������60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������_________(������ȡ��������)�������к���__________(������ȡ��������)
������0.5mol/L��������NaOH�������ʵ�飬��ʵ���в�õ����к�������ֵ��____(����ƫ��������ƫС������������) �� ��������___
����Ŀ������п���㷺Ӧ����ҽҩ�����ũҵ��������ҵ��������п��![]() ��Ҫ�ɷ�ΪZnO������
��Ҫ�ɷ�ΪZnO������![]() ��
��![]() ��CuO��
��CuO��![]() ����
����![]() ��һ��������ͼ��
��һ��������ͼ��
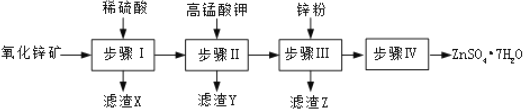
![]() �����IJ�����______������A����Ҫ�ɷ���______��
�����IJ�����______������A����Ҫ�ɷ���______��
![]() ��������ϡ����������ʱ���費��ͨ�����ˮ������Ŀ����______��
��������ϡ����������ʱ���費��ͨ�����ˮ������Ŀ����______��
![]() ������У���pHԼΪ
������У���pHԼΪ![]() ����Һ�м��������أ�����
����Һ�м��������أ�����![]() ��
��![]() ���ֳ������÷�Ӧ�����ӷ���ʽΪ______��
���ֳ������÷�Ӧ�����ӷ���ʽΪ______��
![]() �������˺�������Һ�к��еĽ�����������______��
�������˺�������Һ�к��еĽ�����������______��
![]() ��֪����п���ܽ�����¶�֮��Ĺ�ϵ���±���
��֪����п���ܽ�����¶�֮��Ĺ�ϵ���±���
�¶� | 0 | 20 | 40 | 60 | 80 | 100 |
�ܽ�� |
|
|
|
|
|
|
������п��Һ�л������п�����ʵ�����Ϊ______��______�����ˡ���ɲ������ڼ�ѹ���������½��У�ԭ����______��
![]() ȡ
ȡ![]() g
g![]() ��������ͬ�¶ȣ�ʣ����������仯��ͼ��ʾ��
��������ͬ�¶ȣ�ʣ����������仯��ͼ��ʾ��
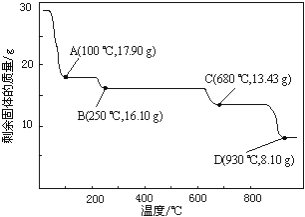
�������ݣ�![]() ʱ���ù���Ļ�ѧʽΪ______��
ʱ���ù���Ļ�ѧʽΪ______��
![]()
![]()
![]()
![]()
����Ŀ������ʵ����������Ӧ�����ӷ���ʽ����ȷ����( )
ѡ�� | ʵ�� | ���� | ���ӷ���ʽ |
A |
| �ڿ����з���һ��ʱ�����Һ����ɫ | 4H++4I��+O2=2I2+2H2O |
B |
| ��Һ��dz��ɫ��Ϊ��ɫ |
|
C |
| ��Һ�ɻ���ɫ��Ϊ��ɫ |
|
D |
| �а�ɫ�������ɣ���Һ�ɺ�ɫ����ɫ |
|
A.AB.BC.CD.D