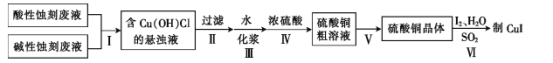
��Ŀ����
����Ŀ����ҵ�Ͽ������ﴦ���� KCN �ķ�ˮ����һ������������������������£��� KCN ת���� KHCO3�� NH3����� pH��6.7��7.2�����ڶ����ǰѰ�ת��Ϊ���NH3+2O2 ![]() HNO3+H2O�� �����������գ�
HNO3+H2O�� �����������գ�
(1) д����һ����Ӧ�Ļ�ѧ��Ӧ����ʽ_____���ڶ�����Ӧ�Ļ�ԭ������________________����д��ѧʽ����
(2) N ԭ�ӡ�H ԭ�ӡ�O ԭ�ӵİ뾶�ɴ�С��˳����____________________��
(3) ̼ԭ�ӵĽṹʾ��ͼΪ________________��ˮ���ӵĵ���ʽΪ_______________________��
(4) �����£�0.1mol/LK2CO3��KCN��KHCO3 ��Һ���ʼ����� pH ���μ�С���� 0.1mol/L �� KCN��KHCO3��K2CO3 ��Һ�У�CN-��HCO3-��CO32-�����ʵ���Ũ���ɴ�С��˳����_____��
(5) ��ҵ�ϻ������������������� KCN �ķ�ˮ��KCN+2KOH+Cl2=KOCN+2KCl+H2O��2KOCN+4KOH+3Cl2=N2+6KCl+2CO2+2H2O��������ȣ����ﴦ�������ŵ���ȱ���ǣ���дһ������ �ŵ㣺__________________��ȱ�㣺__________________��
���𰸡�2KCN+O2+4H2O =2KHCO3+2NH3 HNO3��H2O N>O>H ![]()
![]() c(HCO3-)>c(CN-)>c(CO32-) ������Һ��й¶ ������Ӧ�Բ�
c(HCO3-)>c(CN-)>c(CO32-) ������Һ��й¶ ������Ӧ�Բ�
��������
����Ϊһ�ۺ���ϰ�⣬�漰��������ԭ��Ӧ����ʽ��д�������жϣ����ʽṹ��ԭ�Ӱ뾶�Ƚϡ�ԭ�ӽṹʾ��ͼ�͵���ʽ��д������ˮ�⣬��ҵ�����Ƚϣ�֪ʶ��϶ࡣ
��1����������������������£��� KCN ת���� KHCO3�� NH3�����������غ�͵����غ�ɵ÷���ʽΪ��2KCN+O2+4H2O =2KHCO3+2NH3���ݻ��ϼ۱仯��Ԫ����0�۱�Ϊ-2�ۿ�֪�ڶ�����Ӧ����������������ԭ������HNO3��H2O��
��2����N��O��H��Ԫ�������ڱ���λֵȷ��ԭ�Ӱ뾶��С��ϵ�� N>O>H��
��3���ݺ�������Ų�����̼��ԭ�ӽṹʾ��ͼΪ�� ����ԭ�ӳɼ�����ˮ�ĵ���ʽΪ��
����ԭ�ӳɼ�����ˮ�ĵ���ʽΪ��![]()
(4). �����ζ��������Σ�������ˮ����Һ�Լ��ԣ���Ũ�ȵ��ε�PHֵԽС�������ӵ�ˮ��̶�ԽС������������Ũ��Խ�����������ӵ�Ũ�ȴ�С˳��Ϊ��c(HCO3-)>c(CN-)>c(CO32-)��
��5�������ַ�������ԭ��ȷ���ŵ㣺������Һ��й¶��ȱ�㣺������Ӧ�Բ

����Ŀ��ʵ����������80mL0.1mol/L��̼������Һ����ղ��ش��������⣺
��1������80mL1.0mol/L��Na2CO3��Һ��
ʵ��Ӧ��Na2CO3����/g | Ӧѡ������ƿ�Ĺ��/mL |
___ | ___ |
��2������ʱ������ȷ�IJ���˳��Ϊ___������ĸ��ʾ��ÿ����ĸֻ����һ�Σ���
A.��ȷ������Na2CO3���嵹���ձ��У��ټ�����ˮ�ܽ�
B.������ƿ�ǽ������µߵ���ҡ��
C.������ȴ����Һ�ز�����ע������ƿ��
D.��30 mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
E������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1~2cm��
F����Ϊ��ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�������
��3�������������������������ҺŨ�Ƚ��к�Ӱ�죿(�ƫ�ߡ���ƫ�͡�����Ӱ�족)
������ʱ���ӿ̶��ߣ������___��
������ƿ������������ˮ�������___��
����Ŀ����1.013��105 Pa�£���õ�ijЩ�����ķе���±����ݱ�����������˵����ȷ����(����)
�������� | �е�/�� |
������CH3(CH2)2CH3 | ��0.5 |
������CH3(CH2)3CH3 | 36.1 |
������ | 27.8 |
������ | 9.5 |
������CH3(CH2)4CH3 | 69.0 |
A. �ڱ�״��ʱ��������������
B. ��1.013��105 Pa��20 ��ʱ��C5H12����Һ��
C. ������̼ԭ�������ӣ��е㽵��
D. C5H12��֧�����ӣ��е㽵��