��Ŀ����
����Ŀ���κ����ʵ�ˮ��Һ������ˮ�ĵ���ƽ�⣬����뷽��ʽ�ɱ�ʾΪ��![]() ���±��Dz�ͬ�¶���ˮ�����ӻ����ݣ�
���±��Dz�ͬ�¶���ˮ�����ӻ����ݣ�
�¶ȣ��棩 | 25 |
|
|
ˮ�����ӻ����� |
| a |
|
���������գ�
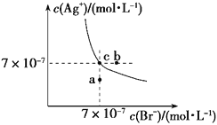
��1��25��ʱ����100mL��ˮ�м���0.01mol��![]() ���壬________��ѡ��ٽ��������ơ�����ˮ�ĵ���ƽ�⣬������Һ��________�ԣ�ѡ��ᡱ��������С�����ԭ���ǣ������ӷ�Ӧ����ʽ��ʾ��________________________��
���壬________��ѡ��ٽ��������ơ�����ˮ�ĵ���ƽ�⣬������Һ��________�ԣ�ѡ��ᡱ��������С�����ԭ���ǣ������ӷ�Ӧ����ʽ��ʾ��________________________��
��2����![]() ����a________
����a________![]() ��ѡ�
��ѡ�![]() ������
������![]() ����=������������____________��
����=������������____________��
��3��![]() ��ʱ����ô�ˮ��
��ʱ����ô�ˮ��![]() ����
����![]() ________mol/L�����¶���ij������Һ��
________mol/L�����¶���ij������Һ��![]() ������Һ��
������Һ��![]() ________mol/L��
________mol/L��
��4��![]() ��ʱ��0.01mol/L��NaOH��Һ��
��ʱ��0.01mol/L��NaOH��Һ��![]() ________��
________��
���𰸡��ٽ� �� ![]()
![]() ˮ�ĵ��������ȷ�Ӧ�����£�ƽ�������ƶ���
ˮ�ĵ��������ȷ�Ӧ�����£�ƽ�������ƶ���![]() ��
��![]() ������
������![]() ��
��![]() ����
���� ![]()
![]() 10
10
��������
��1���Ȼ��ˮ�������ԣ�
��2��ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룬����ˮ�����ӻ���������
��3���ڵ���ƽ�⣬�������������Ũ�Ⱥ�����������Ũ����ͬ������ش�
��4������pH= -lgc(H+)�����㡣
��1��25��ʱ����100mL��ˮ�м���0.01mol��![]() ���壬�ٽ�ˮ�ĵ��룬笠�����ˮ�������ԣ���ˮ�ⷽ��ʽΪ��
���壬�ٽ�ˮ�ĵ��룬笠�����ˮ�������ԣ���ˮ�ⷽ��ʽΪ��![]() ��
��
��2��ˮ�ĵ���Ϊ���ȷ�Ӧ�������¶ȣ�ƽ����������Ӧ�����ƶ�������ˮ�����ӻ����������ݱ���֪���¶ȴ�С˳���ǣ�25��t1��t2����a��1��1014��
��3��ij�¶��´�ˮ�е�c(H+)=2.4��107mol/L�����ʱ��Һ�е�c(OH)=2.4��107mol/L�����¶���ˮ�����ӻ���Kw= c(H+)��c(OH)=2.4��107mol/L��2.4��107 mol/L=5.76��1014��ij������Һ��![]() ����������Ũ��Ϊ102mol/L�������Һ��c(OH)=
����������Ũ��Ϊ102mol/L�������Һ��c(OH)=![]() =
=![]() =5.76��1012 molL1��
=5.76��1012 molL1��
��4��0.01mol/L������������Һ���������ӵ�Ũ��c(OH)=102mol/L����c(H+)=![]() =10-10mol/L��pH= lgc(H+)= lg1010=10��
=10-10mol/L��pH= lgc(H+)= lg1010=10��

����Ŀ�������ˮ��Һ�д��ڵ���ƽ�⡢ˮ��ƽ�⡢�ܽ�ƽ�⣬��ش��������⡣
��1����֪��������ĵ��볣�����±���
���� | HCOOH | HCN | H2CO3 |
���볣��(25��) | Ka = 1��77��10 -4 | Ka=4.3��l0-10 | Ka1=5.0��l0-7 Ka2=5.6��l0-11 |
��0.1 moI/L NaCN��Һ��0.1mol/L NaHCO3��Һ�У�c��CN-��______c��HCO3 -��������>������<������=������
�ڳ����£�pH��ͬ��������Һ
A��HCOONa B��NaCN C��Na2CO3��
�����ʵ���Ũ���ɴ�С��˳����________�����ţ���
����֪25��ʱ��HCOOH( aq) +OH -( aq)=HCOO-(aq) +H2O��1�� ��H=-a kJ/mol
H+(aq) +OH-(aq) =H2O��1�� ��H=-b kJ/mol
���������Ȼ�ѧ����ʽΪ__________________________________��
�ܽ�����CO2ͨ��NaCN��Һ����Ӧ�����ӷ���ʽ��______________________��
�������£�����Ũ�ȵ�HCOONa��ҺpH =9�������ӷ���ʽ��ʾ��Һ�ʼ��Ե�ԭ����:
______________________________����Һ��![]() =___________��
=___________��
��2�������£���0.100 mol/L������Һ�ζ�20.00mL0.l00mol/L ��ij��ˮ��Һ���ζ�������ͼ��ʾ��
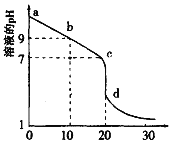
��d����ʾ����Һ������Ũ���ɴ�С��˳������Ϊ_______________��
��b����ʾ����Һ��c(NH3��H2O)-c(NH4+)=_____������Һ�е���������Ũ�ȱ�ʾ����
��pH =10�İ�ˮ��pH =4��NH4C1��Һ�У���ˮ�������c(H+)֮��Ϊ____��
��3����֪Ksp(BaCO3) =2.6��l0-9��Ksp( BaSO4)=1.1��10-10.
���ֽ�Ũ��Ϊ2��10-4mol/LNa2CO3��Һ��BaCl2��Һ�������ϣ�������BaCO3��������BaCl2��Һ����СŨ��Ϊ____mol/L��
������BaSO4�������Һ�еμ�Na2CO3��Һ������BaCO3��������ʱ����Һ��![]() =___________��������λ��Ч���֣���
=___________��������λ��Ч���֣���