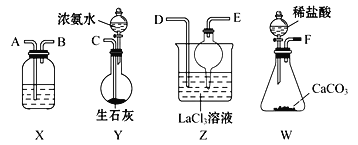
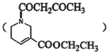
��Ŀ����
����Ŀ����������ʮ�����ʣ���NaHCO3����C2H5OH����Cu����H2O����ʯ���飻��CO����Ba(OH)2���������H2CO3����Ũ���ᡣ
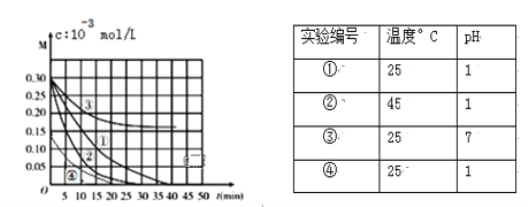
��1�����ڵ���ʵ���___����д��ţ������ڷǵ���ʵ���___����д��ţ���
��2������ˮ��Һ�еĵ��뷽��ʽΪ___��
��3������෴Ӧ�����ӷ���ʽΪ___��
��4����ߵ���Һ�еμӢٵ���Һ��Ba2+ǡ����ȫ���������ӷ���ʽΪ___��
��5������ⷴӦ�����ӷ���ʽ���£�����ƽ����ʽ����������ϵ�����ں�����д��ȱ�ٵ����ʣ������������������������ת�Ƶķ�������Ŀ��___
Cu+NO3��+ ��Cu2++NO2��+
���𰸡��٢ܢߢ� �ڢ� H2CO3![]() H++HCO3����HCO3��
H++HCO3����HCO3��![]() H++CO32- Ca(OH)2+2H+ �TCa2++2H2O Ba2++HCO3��+OH���TBaCO3��+H2O
H++CO32- Ca(OH)2+2H+ �TCa2++2H2O Ba2++HCO3��+OH���TBaCO3��+H2O 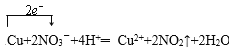
��������
��NaHCO3���Σ����ڵ���ʣ�
��C2H5OH���л�����ڷǵ���ʣ�
��Cu���ڽ������ʣ��Ȳ��ǵ����Ҳ���Ƿǵ���ʣ�
��H2O�Ƿǽ�����������ڵ���ʣ�
��ʯ������������������Һ��Ϊ�����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ�
��CO�Ƿǽ�����������ڷǵ���ʣ�
��Ba(OH)2�Ǽ���ڵ���ʣ�
������Ϊ�����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ�
��H2CO3���ᣬ���ڵ���ʣ�
��Ũ����Ϊ�����Ȳ��ǵ����Ҳ���Ƿǵ���ʡ�
��1���ɷ�����֪�����ڵ���ʵ����٢ܢߢ������ڷǵ���ʵ����ڢ����ʴ�Ϊ���٢ܢߢ����ڢ���
��2��H2CO3�Ƕ�Ԫ���ᣬ��ˮ��Һ�зֲ����룬���뷽��ʽΪH2CO3![]() H++HCO3����HCO3��
H++HCO3����HCO3��![]() H++CO32-���ʴ�Ϊ��H2CO3
H++CO32-���ʴ�Ϊ��H2CO3![]() H++HCO3����HCO3��
H++HCO3����HCO3��![]() H++CO32-��
H++CO32-��
��3��ʯ���������ᷴӦ�����Ȼ��ƺ�ˮ��ʯ������������������Һ�����ܲ�д����Ӧ�����ӷ���ʽΪCa(OH)2+2H+ �TCa2++2H2O���ʴ�Ϊ��Ca(OH)2+2H+ �TCa2++2H2O��
��4��������������Һ�еμ�̼�����Ƶ���Һ��Ba2+ǡ����ȫ����ʱ����Ӧ����̼�ᱵ�������������ƺ�ˮ����Ӧ�����ӷ���ʽΪBa2++HCO3��+OH���TBaCO3��+H2O���ʴ�Ϊ��Ba2++HCO3��+OH���TBaCO3��+H2O��
��5��ͭ��Ũ���ᷴӦ��������ͭ������������ˮ����Ӧ�Ļ�ѧ����ʽΪCu+4HNO3�TCu��NO3��2+2NO2��+2H2O����Ӧ��ת��2mol���ӣ�����ת�Ƶķ�������ĿΪ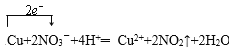 ���ʴ�Ϊ��
���ʴ�Ϊ��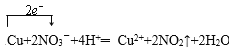 ��
��

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�����Ŀ��ѧϰС���о��Ƶ�ȼ�շ�Ӧ���ȡ������(�����ѱ�����)������ú�ͣ����������ڣ��Ϸ�����ʢ��O2����ƿ������һ��ʱ�䣬��ַ�Ӧ��۲쵽����Ϊ��ɫ����͵���ɫ����Ļ�����÷�Ӧǰ������ʵ��������±���
����/g | ||
��Ӧǰ | ���� | 100 |
ʢ�н����Ƶ����� | 105.4 | |
��Ӧ�� | ʢ�й����������� | 107.4 |
��ش��������⣺
(1)����ʵ�������Ʋ�������ɷ�Ϊ___________________���ѧʽ��
(2)������ȫ������ˮ���۲쵽���������ɣ�д���÷�Ӧ�Ļ�ѧ����ʽΪ________��
(3)���������Һ��n(Na+)=0.2mol��ͨ������֤ʵ���ȹ����д��ڱ����Na2Oת��ΪNa2O2��___________________