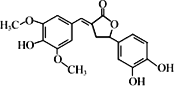
��Ŀ����
����Ŀ���л���A��C6H8O4��ΪʳƷ��װֽ�ij��÷�������A����ʹ��ˮ��ɫ��A������ˮ���������������¿ɷ���ˮ�ⷴӦ���õ�B(C4H4O4)�ͼ״���ͨ��״����BΪ��ɫ���壬��������������Һ������Ӧ��
��1��A���Է����ķ�Ӧ��_______________��ѡ����ţ���
�ټӳɷ�Ӧ ��������Ӧ �ۼӾ۷�Ӧ ��������Ӧ
��2��B�������������ŵ�������______________________________��
��3��B������û��֧������ṹ��ʽ��________________________��B�ľ�����ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ��_________________��
��4����B��ȡA�Ļ�ѧ����ʽ��____________________________________��
��5�����Ŷ����ᣨC4H7NO4����������嵰���ʵİ�����֮һ������Bͨ�����·�Ӧ��ȡ��
![]()
���Ŷ�����Ľṹ��ʽ��______________________________________��
���𰸡��٢ۢ� ̼̼˫�����Ȼ� HOOCCH=CHCOOH CH2=C(COOH)2 HOOCCH=CHCOOH+2CH3OH![]() CH3OOCCH=CHCOOCH3+2H2O
CH3OOCCH=CHCOOCH3+2H2O 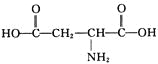
��������
A�ķ���ʽΪC6H8O4��A�IJ����Ͷ�Ϊ3��A����ʹ��ˮ��ɫ��A�к�̼̼˫����A�����������¿ɷ���ˮ�ⷴӦ�õ�B��C4H4O4����CH3OH��B��������������Һ��Ӧ��B�к������Ȼ���̼̼˫����A�к�����������
��1��A�к���̼̼˫����������A�к�̼̼˫����A�ܷ����ӳɷ�Ӧ���Ӿ۷�Ӧ��������Ӧ��A�в������ǻ����Ȼ���A���ܷ���������Ӧ����ѡ�٢ۢܡ�
��2��B����������������Ϊ̼̼˫�����Ȼ���
��3��B������û��֧����B�Ľṹ��ʽΪHOOCCH=CHCOOH��B�ľ�����ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪCH2=C(COOH)2��
��4��B��CH3OH����������Ӧ����A����B��ȡA�Ļ�ѧ����ʽΪHOOCCH=CHCOOH+2CH3OH![]() CH3OOCCH=CHCOOCH3+2H2O��
CH3OOCCH=CHCOOCH3+2H2O��
��5��B�Ľṹ��ʽΪHOOCCH=CHCOOH��B��HCl�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪHOOCCH2CHClCOOH��C��NH3��Ӧ�������Ŷ����ᣬ���Ŷ����ᣨC4H7NO4����������嵰���ʵİ�����֮һ�������Ŷ�����Ľṹ��ʽΪ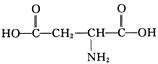 ��
��

����Ŀ���ᾧ������������ʧˮʱ�������������FeSO4��7H2O�������������㣬��ֵ�ᳬ��100�������ұ��涨��FeSO4��7H2O�ĺ�����һ��Ʒ99.50����100.5��������Ʒ99.00����100.5��������Ʒ98.00����101.0����
Ϊ�ⶨ��Ʒ��FeSO4��7H2O�������������ɲ�������������������������Һ���еζ���
5Fe2����MnO4����8H����5Fe3����Mn2����4H2O��
2MnO4����5C2O42����16H����2Mn2����10CO2����8H2O
�ⶨ���̣�����һ��Ũ�ȵĸ��������Һ1L��Ȼ���ȡ0.200 g ����Na2C2O4��ʽ��Ϊ134.0��������ƿ�У�������ˮ�ܽⲢ��ϡ�����ữ��������70�桫80�档
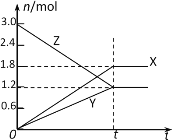
(1)��Ҫ�õζ����ⶨ����ĸ��������ҺŨ�ȣ��ζ��յ��������_______________��
(2)����Һ���ȵ�Ŀ����____����Ӧ�տ�ʼʱ��Ӧ���ʽ�С���������¶�����Ӱ���������Ӱ�컯ѧ��Ӧ���ʵ�������������ԭ�������______________________��
(3)���ζ�ʱ���ֵζ��ܼ��첿�������ݣ��ζ�����������ʧ�����ø������Ũ��_____������ƫ������ƫС������Ӱ��������
(4)�ζ���ȥ���������Һ29.50mL����c(KMnO4)��_____mol/L��������λ��Ч���֣���
(5)��ȡ�ķ�FeSO4��7H2O������������Ϊ0.506g�������������������Һ�ζ��ﵽ�յ㣬��¼�ζ�����
�ζ����� ʵ������ | 1 | 2 | 3 | 4 |
V(�������)/mL(������) | 0.10 | 0.20 | 0.00 | 0.20 |
V(�������)/mL(�ն���) | 17.76 | 17.88 | 18.16 | 17.90 |
��������FeSO4��7H2O�ĺ���������������Ϊ_________��С���������λ�������Ϲ���______������
(6)��ʵ��ȷֵΪ99.80%��ʵ��������=____%��������в����Լ����������յ��жϵ�ʧ�����������Ŀ���ԭ���ǣ�__________��
����Ŀ���������ȣ�ClO2����һ�ָ�Ч��������������ˮ���е�Ϊ11.0�������ױ�ը���ڸ������ϡ�������£��ø����������������������Ʊ��������ȣ�װ����ͼ��
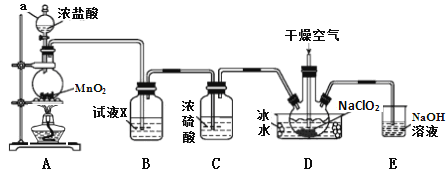
(1)����a������Ϊ_____________��װ��A�з�Ӧ�����ӷ���ʽΪ_______________��
(2)�Լ�X��_______________________��
(3)װ��D�б�ˮ����Ҫ������___________��װ��D�ڷ�����Ӧ�Ļ�ѧ����ʽΪ_______________��
(4)װ��E����Ҫ��Ӧ�����ӷ���ʽΪ��____________________________��
(5)��֪NaClO2������Һ�ڲ�ͬ�¶�ʱ�����ľ���������±���
�¶� | ��38�� | 38����60�� | ��60�� |
�������� | NaClO2��3H2O | NaClO2 | �ֽ��NaClO3��NaCl |
����NaClO2��Һ�Ƶ�NaClO2����IJ������裺 55�������ᾧ��_________��38��60������ˮϴ�ӡ�����60�����
(6)��ҵ��Ҳ�������·����Ʊ�ClO2��
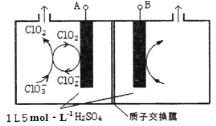
������������˫��ˮ��NaClO3��Ӧ����Ӧ�����ӷ���ʽΪ_______________________��
����ͼ��ʾΪֱ�ӵ�������ơ��Զ���ѭ���Ʊ��ߴ�ClO2��ʵ�顣�������缫��ӦʽΪ____________��