��Ŀ����
��13�֣�������������Ԫ��A��B��C��D��Eԭ������������������A��B��Cԭ�Ӻ�����Ӳ���֮����5����Cԭ��������ϵĵ�����ΪA��B��Ԫ��ԭ��������Ӳ��ϵ��������ܺͣ�Cԭ�ӵļ۵��ӹ���Ϊ![]() ��Aԭ�ӵ�������������������Ӳ�����D��E�����γ�ԭ�Ӹ�����Ϊ1:1��1:2���������ӻ������Dԭ�ӵ�2p�ܼ���������δ�ɶԵ��ӣ��Իش��������⣺
��Aԭ�ӵ�������������������Ӳ�����D��E�����γ�ԭ�Ӹ�����Ϊ1:1��1:2���������ӻ������Dԭ�ӵ�2p�ܼ���������δ�ɶԵ��ӣ��Իش��������⣺
(1)B��C��D��E����Ԫ�ص�ԭ�ӣ��뾶�Ӵ�С��˳���� (��Ԫ�ط��Ż�ѧʽ����ͬ)����һ�����ܴӴ�С��˳����
(2)Cԭ�ӵĵ����Ų�ͼ_____________,D2-�Ľṹʾ��ͼ___________��A��D���γɶ��ֻ��������һ��������������ˮ��Һ�������ԣ�д���û�����Ľṹʽ________________,�����к���_____________________��������ԡ��Ǽ��ԡ�����ͬ����__________���ӡ�
(3) A��B��D����Ԫ�ؿ����γɶ����л���������ӣ��������ԭ�������ٵ�һ��������װ��ʱ�γɵ���Ҫ������Ⱦ���д�����ĵ���ʽ_ _�����ݼ۲���ӶԻ��⣨VSEPR�������Ʋ�÷�������ԭ�ӵ��ӻ���ʽΪ �ӻ����ռ乹��Ϊ__ ��
(4) C��E�γ�һ�����ӻ�����Y����Ħ������Ϊ65g/mol��Y���Ȼ�ײ��ʱ�����ֽ�Ϊ���ֵ��ʡ���Y�Ļ�ѧʽΪ ��Y����������һ����������ӣ�ȫ��CԪ����ɣ�Y�����ᷴӦ�����ӷ���ʽΪ ��
����13��ÿ��1�֣������ӷ�Ӧ��
(1)Na>C>N>O�� N>O>C>Na
(2) �� ��H-O-O-H �����Լ��ͷǼ��Լ� ������
��3���� SP2 ƽ��������
(4)![]() ;
; ![]() ��2�֣�
��2�֣�
����:��

 ��Aԭ�ӵ�������������������Ӳ�����D��E�����γ�ԭ�Ӹ�����Ϊ1:1��1:2���������ӻ������Dԭ�ӵ�2p�ܼ���������δ�ɶԵ��ӣ��Իش��������⣺
��Aԭ�ӵ�������������������Ӳ�����D��E�����γ�ԭ�Ӹ�����Ϊ1:1��1:2���������ӻ������Dԭ�ӵ�2p�ܼ���������δ�ɶԵ��ӣ��Իش��������⣺ ѧʽ����ͬ)����һ�����ܴӴ�С��˳����
ѧʽ����ͬ)����һ�����ܴӴ�С��˳����  _____________,D2-�Ľṹʾ��ͼ___________��A��D���γɶ��ֻ��������һ��������������ˮ��Һ�������ԣ�д���û�����Ľṹʽ________________,�����к���_____________________��������ԡ��Ǽ��ԡ�����ͬ����__________���ӡ�
_____________,D2-�Ľṹʾ��ͼ___________��A��D���γɶ��ֻ��������һ��������������ˮ��Һ�������ԣ�д���û�����Ľṹʽ________________,�����к���_____________________��������ԡ��Ǽ��ԡ�����ͬ����__________���ӡ�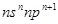 ��Aԭ�ӵ�������������������Ӳ�����D��E�����γ�ԭ�Ӹ�����Ϊ1:1��1:2���������ӻ������Dԭ�ӵ�2p�ܼ���������δ�ɶԵ��ӣ��Իش��������⣺
��Aԭ�ӵ�������������������Ӳ�����D��E�����γ�ԭ�Ӹ�����Ϊ1:1��1:2���������ӻ������Dԭ�ӵ�2p�ܼ���������δ�ɶԵ��ӣ��Իش��������⣺