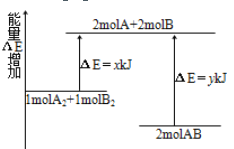
��Ŀ����
����Ŀ�����ᣨH2C2O4����һ�ֶ�Ԫ���ᣬ�ڻ�ѧ���������Ҫ����;��ijʵ��Ա��Ҫ�ò��ᾧ�壨H2C2O42H2O������400mL 0.4mol/L��H2C2O4��Һ����ش���������
��1��ͨ�����㣬��ʵ��ԱӦ����������ƽ��______ g H2C2O42H2O������ʵ�顣
��2����д����ʵ���ʵ�鲽��
a:���㣬b������c:_____��d:��ȴ,e:��Һf:ϴ��g:________��h:ҡ�ȣ�i:װҺ��
��3���ڴι������õ��IJ����������˲��������ձ�����ͷ�ι��⣬����_____��
��4���������ƹ����У�����ʵ������������Ƶ���Һ�����ʵ���Ũ���к�Ӱ�죿��ѡ�ƫ�ߡ���ƫ�͡�����Ӱ�족����
I��������ĩʱ���������λ�÷ŷ�����___________��
II����Һ���̺�û�жԲ��������ձ�����ϴ����_________��
III.����ʱ���ӿ̶�����___________��
IV.ҡ��ʱ������ƿҺ����ڿ̶��ߣ��μ�����ˮ���̶�����_________��
���𰸡�25.2 �ܽ� ���� 500mL����ƿ ƫ�� ƫ�� ƫ�� ƫ��
��������
����һ��������Ũ�ȵIJ��裺1.���㣻2.������3.�ܽ⣻4.��ȴ��5.��Һ��6.ϴ�ӣ�7.���ݣ�8.ҡ�ȣ�9.װƿ���õ��������У�������ƽ��ҩ�ס��ձ���Ͳ����������������ƿ����ͷ�ιܣ�����n=cV����������������������������������ݸ���c= ![]() �ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V ����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V �����ı仯���Դ˽��
�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V ����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V �����ı仯���Դ˽��
��1��ʵ����������400mL0.4mol/L��H2C2O4��Һ����Ҫѡ��500mL����ƿ��ʵ�������Ƶ���500mL 0.4mol/L��H2C2O4��Һ����ҪH2C2O4�����ʵ���Ϊ��0.4mol/L��0.5L=0.2mol����ҪH2C2O42H2O�����ʵ���Ҳ��0.2mol������Ϊ��126g/mol��0.2mol=25.2g��
��2������500mL 0.4mol/L��H2C2O4��Һ�IJ��裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װҺ��
��3������һ�����ʵ���Ũ����Һһ�㲽��Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�ȣ��õ��������У�������ƽ��ҩ�ס��ձ���Ͳ����������������ƿ����ͷ�ιܣ�ȱ�ٵ�����Ϊ��500mL����ƿ��
��4��I.��������̷����壬���̷����룬����֪��m(����)+m(����)=m(����)����֪�������Ƶ�������ʵ��Ҫ�Ƶ�����Ҫ�ᣬ��ʹ������Һ�����ʵ���Ũ��ƫ�ͣ�
II����Һ���̺�û�жԲ��������ձ�����ϴ�ӣ�����������ʧ����ʹ������Һ�����ʵ���Ũ��ƫ�ͣ�
III.����ʱ���ӿ̶��ߣ��������Һ���ƫС����ʹ������Һ�����ʵ���Ũ��ƫ�ߣ�
IV.ҡ��ʱ������ƿҺ����ڿ̶��ߣ��μ�����ˮ���̶��ߣ��������Һ���ƫ��ʹ������Һ�����ʵ���Ũ��ƫ�͡�

 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�