��Ŀ����
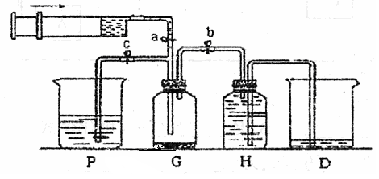
ij����С��������ͼ��ʾװ����ȡ�������ṩ���Լ��У�Ũ���ᡢ����ʳ��ˮ������������Һ��������ع��塣��Ӧ�Ļ�ѧ����ʽΪ��
2KMnO4��+ 16HCl��Ũ���� 2KCl + 2MnCl2��+ 5Cl2��+ 8H2O
�Իش��������⣺
��1���ڷ�Ӧ2KMnO4 + 16HCl��Ũ���� 2KCl + 2MnCl2 + 5Cl2��+ 8H2O�У�HCl���ֵ�����Ϊ________��_________.�����ɵ�Cl2�����5.6L����״���£�,��ת�Ƶĵ��ӵĸ���________(����٤��������NA����ʾ)
��2��װ��H��ʢ�ŵ��Լ��� ��װ��P��ʢ�ŵ��Լ��� ��
��3��β������ʱ�رյ��ɼ�a�͵��ɼ� �����ɼ� ��
��4������β��ʱ��������Ӧ�����ӷ���ʽΪ ��
2KMnO4��+ 16HCl��Ũ���� 2KCl + 2MnCl2��+ 5Cl2��+ 8H2O

�Իش��������⣺
��1���ڷ�Ӧ2KMnO4 + 16HCl��Ũ���� 2KCl + 2MnCl2 + 5Cl2��+ 8H2O�У�HCl���ֵ�����Ϊ________��_________.�����ɵ�Cl2�����5.6L����״���£�,��ת�Ƶĵ��ӵĸ���________(����٤��������NA����ʾ)
��2��װ��H��ʢ�ŵ��Լ��� ��װ��P��ʢ�ŵ��Լ��� ��
��3��β������ʱ�رյ��ɼ�a�͵��ɼ� �����ɼ� ��
��4������β��ʱ��������Ӧ�����ӷ���ʽΪ ��
��b��c��1�֣������Ϊ2�֣���14�֣�
��1�����Ժͻ�ԭ�ԣ�0.5NA, ��2������ʳ��ˮ������������Һ
��3��b c ��4��Cl2��2OH��=Cl����ClO����H2O
��1�����Ժͻ�ԭ�ԣ�0.5NA, ��2������ʳ��ˮ������������Һ
��3��b c ��4��Cl2��2OH��=Cl����ClO����H2O
�����������1���ڷ�Ӧ�У���Ԫ�صĻ��ϼ۴ӣ�1�����ߵ�0�ۣ����������Ȼ���Ļ�ԭ�ԡ���ͬʱ�����Ȼ������ɣ������Ȼ�������ԡ��������������ʵ�����5.6L��22.4L/mol��0.25mol�������ڷ�Ӧ��ת�Ƶ��ӵ����ʵ�����0.25mol��2��0.5mol����ת�Ƶĵ��ӵĸ�����0.5NA��
��2����������������ˮ�����Կ������ű���ʳ��ˮ���ռ���������H��ʢ�ŵ��DZ���ʳ��ˮ��
��3��P��ʢ�ŵ�����������Һ����������β��������β������ʱ�رյ��ɼ�a�͵��ɼ�b�����ɼ�c��
��4������������������Һ��Ӧ�����ӷ���ʽ��Cl2��2OH��=Cl����ClO����H2O��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬�����ۺ���ǿ���������С���ע�ض�ѧ��������֪ʶ�����ѵ����ͬʱ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ�����Ͻ��淶��ʵ�����������Ҳ���������ѧ����ѧ������������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

��ϰ��ϵ�д�
�����Ŀ




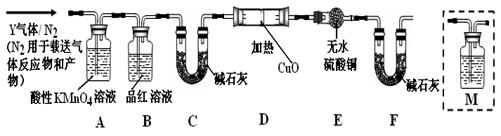

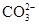 ���μ�ϡ���ᣬ������������ͨ�����ʯ��ˮ
���μ�ϡ���ᣬ������������ͨ�����ʯ��ˮ ���ȵμ�ϡ���ᣬ�ٵμ�BaCl2��Һ
���ȵμ�ϡ���ᣬ�ٵμ�BaCl2��Һ