��Ŀ����
��֪N2O4����ɫ�� 2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�| ʱ�䣨S�� | 20 | 40 | 60 | 80 | 100 | |
| n�� N2O4 ����mol�� | 0.80 | a | 0.40 | c | d | e |
| n��NO2����mol�� | 0.00 | 0.48 | b | 1.04 | 1.20 | 1.20 |
��2����Ӧ�ﵽƽ����¶�������100�棬������ɫ�����÷�Ӧ���淴Ӧ��______������ȡ������ȡ���
��3���÷�Ӧ��ƽ�ⳣ����ʾʽΪK=______Ҫ����÷�Ӧ��Kֵ���ɲ�ȡ�Ĵ�ʩ�У�����ţ�______��
��A������������ͨ��NO2 ��B��ʹ�ø�Ч����
��C�������¶� ��D������N2O4����ʼŨ��
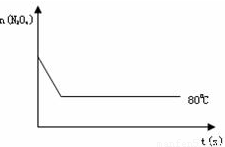
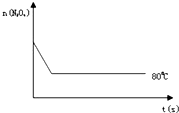
��4����ͼ��80��ʱ������N2O4���ʵ����ı仯���ߣ����ڸ�ͼ�в������÷�Ӧ��100��ʱN2O4���ʵ����ı仯���ߣ���Ӧ����ʼ�����ʵ�����ȣ���
���𰸡���������1�����ݱ������ݿɵø�ʱ��ʱ��Ӧ�������������ʵ������Դ˼��㷴Ӧ���ʺ�ת���ʣ�
��2����Ӧ�ﵽƽ����¶�������100�棬������ɫ���˵�������¶�ƽ��������Ӧ�����ƶ���
��3����Ӧ��ƽ�ⳣ��Ϊ�������Ũ����֮�����Է�Ӧ���Ũ����֮����Ҫ����÷�Ӧ��Kֵ��Ӧʹƽ��������Ӧ�����ƶ���
��4�������¶ȷ�Ӧ��������ƽ�����ƣ�ƽ��ʱN2O4�����ʵ�����80��ʱ�٣��Դ˻�ͼ��
����⣺��1�����ݱ������ݿɵø�ʱ��ʱ��Ӧ�������������ʵ�����
N2O4����ɫ�� 2NO2
2NO2
0sʱ��0.80mol 0
20sʱ��a 0.48mol
40sʱ��0.40mol b
80sʱ��0.20mol 1.20mol
��a=0.80mol-0.24mol=0.56mol��b=0.80mol��
��20s-40s����NO2��ʾ��ƽ����Ӧ����Ϊ =0.004mol/��L?S�����ɱ������ݿ�֪������Ӧ�ﵽ80sʱ��Ӧ�ﵽƽ��״̬��ƽ��ʱN2O4��ת����Ϊ
=0.004mol/��L?S�����ɱ������ݿ�֪������Ӧ�ﵽ80sʱ��Ӧ�ﵽƽ��״̬��ƽ��ʱN2O4��ת����Ϊ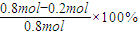 =75%��
=75%��
�ʴ�Ϊ��0.004mol/��L?S����75%��
��2����Ӧ�ﵽƽ����¶�������100�棬������ɫ���˵�������¶�ƽ��������Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ���淴ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
��3����Ӧ��ƽ�ⳣ��Ϊ�������Ũ����֮�����Է�Ӧ���Ũ����֮������ΪK=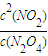 ��Ҫ����÷�Ӧ��Kֵ��Ӧʹƽ��������Ӧ�����ƶ���ͨ�� N2O4��NO2���൱����ԭ���Ļ�������С�������ѹǿ����ƽ���������ƶ���Kֵ��С�����������ƽ��û��Ӱ�죬�����¶�ƽ�����ƣ�Kֵ����
��Ҫ����÷�Ӧ��Kֵ��Ӧʹƽ��������Ӧ�����ƶ���ͨ�� N2O4��NO2���൱����ԭ���Ļ�������С�������ѹǿ����ƽ���������ƶ���Kֵ��С�����������ƽ��û��Ӱ�죬�����¶�ƽ�����ƣ�Kֵ����
�ʴ�Ϊ��K=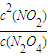 ��C��
��C��
��4�������¶ȷ�Ӧ��������ƽ�����ƣ�ƽ��ʱN2O4�����ʵ�����8��0ʱ�٣��ʴ�Ϊ��ͼ����ͼ��ʾ��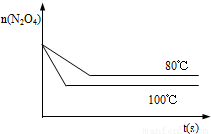
���������⿼�黯ѧƽ���ƶ��Լ��йؼ��㣬��Ŀ��Ϊ�ۺϣ�����һ���Ѷȣ�����ʱע����������Ի�ѧƽ���Ӱ�죮
��2����Ӧ�ﵽƽ����¶�������100�棬������ɫ���˵�������¶�ƽ��������Ӧ�����ƶ���
��3����Ӧ��ƽ�ⳣ��Ϊ�������Ũ����֮�����Է�Ӧ���Ũ����֮����Ҫ����÷�Ӧ��Kֵ��Ӧʹƽ��������Ӧ�����ƶ���
��4�������¶ȷ�Ӧ��������ƽ�����ƣ�ƽ��ʱN2O4�����ʵ�����80��ʱ�٣��Դ˻�ͼ��
����⣺��1�����ݱ������ݿɵø�ʱ��ʱ��Ӧ�������������ʵ�����
N2O4����ɫ��
 2NO2
2NO2 0sʱ��0.80mol 0
20sʱ��a 0.48mol
40sʱ��0.40mol b
80sʱ��0.20mol 1.20mol
��a=0.80mol-0.24mol=0.56mol��b=0.80mol��
��20s-40s����NO2��ʾ��ƽ����Ӧ����Ϊ
 =0.004mol/��L?S�����ɱ������ݿ�֪������Ӧ�ﵽ80sʱ��Ӧ�ﵽƽ��״̬��ƽ��ʱN2O4��ת����Ϊ
=0.004mol/��L?S�����ɱ������ݿ�֪������Ӧ�ﵽ80sʱ��Ӧ�ﵽƽ��״̬��ƽ��ʱN2O4��ת����Ϊ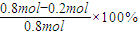 =75%��
=75%���ʴ�Ϊ��0.004mol/��L?S����75%��
��2����Ӧ�ﵽƽ����¶�������100�棬������ɫ���˵�������¶�ƽ��������Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ���淴ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
��3����Ӧ��ƽ�ⳣ��Ϊ�������Ũ����֮�����Է�Ӧ���Ũ����֮������ΪK=
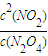 ��Ҫ����÷�Ӧ��Kֵ��Ӧʹƽ��������Ӧ�����ƶ���ͨ�� N2O4��NO2���൱����ԭ���Ļ�������С�������ѹǿ����ƽ���������ƶ���Kֵ��С�����������ƽ��û��Ӱ�죬�����¶�ƽ�����ƣ�Kֵ����
��Ҫ����÷�Ӧ��Kֵ��Ӧʹƽ��������Ӧ�����ƶ���ͨ�� N2O4��NO2���൱����ԭ���Ļ�������С�������ѹǿ����ƽ���������ƶ���Kֵ��С�����������ƽ��û��Ӱ�죬�����¶�ƽ�����ƣ�Kֵ�����ʴ�Ϊ��K=
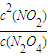 ��C��
��C����4�������¶ȷ�Ӧ��������ƽ�����ƣ�ƽ��ʱN2O4�����ʵ�����8��0ʱ�٣��ʴ�Ϊ��ͼ����ͼ��ʾ��

���������⿼�黯ѧƽ���ƶ��Լ��йؼ��㣬��Ŀ��Ϊ�ۺϣ�����һ���Ѷȣ�����ʱע����������Ի�ѧƽ���Ӱ�죮

��ϰ��ϵ�д�
�����Ŀ
| |||||||||||||||||||||||||||||||||
 2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�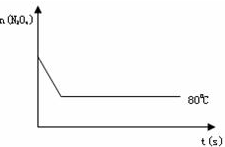
 ��֪N2O4����ɫ��?2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
��֪N2O4����ɫ��?2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�