题目内容
已知:(1)Zn(s)+1/2O2(g)=ZnO(s) △H=-348.3kJ·mol-1 ,
(2)2Ag(s)+1/2O2(g)=Ag2O(s) △H=-31.0kJ·mol-1,
则Zn(s)+Ag2O(s)=ZnO(s)+2Ag(s)的△H等于
(2)2Ag(s)+1/2O2(g)=Ag2O(s) △H=-31.0kJ·mol-1,
则Zn(s)+Ag2O(s)=ZnO(s)+2Ag(s)的△H等于
| A.-317.3kJ·mol-1 | B.-379.3kJ·mol-1 | C.-332.8kJ·mol-1 | D.+317.3kJ·mol-1 |
A
由热化学方程式:(1)Zn(s)+1/2O2(g)=ZnO(s) △H=-348.3kJ·mol-1 ,(2)2Ag(s)+1/2O2(g)=Ag2O(s) △H=-31.0kJ·mol-1,可知,将两热化学方程式叠加,得Zn(s)+Ag2O(s)=ZnO(s)+2Ag(s)△H=-317.3kJ·mol-1

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目
 (2)2Zn(s)+O2(g)=2ZnO(s) Δ
(2)2Zn(s)+O2(g)=2ZnO(s) Δ H1 =" —702" kJ/mol
H1 =" —702" kJ/mol 2Hg(l)+O2(g)=2HgO(s) ΔH2 =" —182" kJ/mol
2Hg(l)+O2(g)=2HgO(s) ΔH2 =" —182" kJ/mol
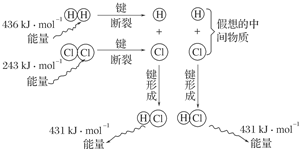
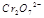 在水溶液中为红色,
在水溶液中为红色,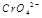 在水溶液中为黄色。某条件下该反应建立平衡后,体系为两种离子的混合液,颜色为橙色。
在水溶液中为黄色。某条件下该反应建立平衡后,体系为两种离子的混合液,颜色为橙色。 ;
;
 ,能有效地利用资源,并减少空气中的温室气体。工业上正在研究利用
,能有效地利用资源,并减少空气中的温室气体。工业上正在研究利用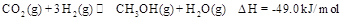
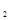 充入一容积为2L的密闭容器中,测得H
充入一容积为2L的密闭容器中,测得H
 (ΔH1+ΔH2)
(ΔH1+ΔH2)