��Ŀ����
����Ŀ�����һ����һ�������ʵ���װ��ͼ����֤������Һ��������̼ˮ��Һ��������Һ������ǿ��˳����CH3COOH��H2CO3��C6H5OH��
(1)������ͼ��ʾ������������װʵ��װ�ã�������������˳����____��D��E��_____��______��_____��
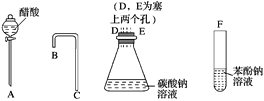
(2)�йط�Ӧ�Ļ�ѧ����ʽΪ________________________________________________��
(3)�е�ͬѧ��Ϊ��װ�ò�����֤H2CO3��C6H5OH������ǿ��������Ϊ________(��С���û�С�)�����������Ľ�ʵ�������֤H2CO3��C6H5OH������ǿ����
________________________________________________________________________��
���𰸡�ABCFNa2CO3��2CH3COOH�D��2CH3COONa��H2O��CO2����![]() ��H2O��CO2�D��
��H2O��CO2�D��![]() ��NaHCO3��Ӧ����ƿ���Թ�֮���һ��ʢ����NaHCO3��Һ��ϴ��ƿ����ȥCO2�л��еĴ�������
��NaHCO3��Ӧ����ƿ���Թ�֮���һ��ʢ����NaHCO3��Һ��ϴ��ƿ����ȥCO2�л��еĴ�������
��������
��ǿ��������ȡ�������ᣬ������������ȡ�������ᣬ�Դ�����������1��Bװ����Ӧʢ�����������ʵ�ҩƷ���Ҳ��ܵ��������������µ����ʣ���2���������ܺͶ�����̼��ˮ��Ӧ���ɱ��Ӻ�̼��������
��1��������Һ��������̼ˮ��Һ��������Һ������ǿ��˳����CH3COOH��H2CO3��C6H5OH������ǿ����ȡ����֪�������̼���Ʒ�Ӧ��ȡ������̼��������̼�ͱ�������Һ��Ӧ��ȡ���ӣ���������������˳���ǣ�A��D��E��A��B��C��F���ʴ�Ϊ��A��B��C��F����2��̼���ƺʹ��ᷴӦ����ʽΪ��Na2CO3��2CH3COOH�D��2CH3COONa��H2O��CO2����������̼�ͱ�������Һ��Ӧ����ʽΪ![]() ��H2O��CO2�D��
��H2O��CO2�D��![]() ��NaHCO3����3�������ӷ��������Ʊ���ǣ������Ǵ����뱽���Ʒ�Ӧ��Ӧ���һ��װ�ó�ȥCO2�л��еĴ������������Ը�ͬѧ˵���е�����Ӧ����ƿ���Թ�֮���һ��ʢNaHCO3��Һ��ϴ��ƿ����ȥCO2�л��еĴ����������ʴ�Ϊ����ͬѧ˵���е�����Ӧ����ƿ���Թ�֮���һ��ʢNaHCO3��Һ��ϴ��ƿ����ȥCO2�л��еĴ���������
��NaHCO3����3�������ӷ��������Ʊ���ǣ������Ǵ����뱽���Ʒ�Ӧ��Ӧ���һ��װ�ó�ȥCO2�л��еĴ������������Ը�ͬѧ˵���е�����Ӧ����ƿ���Թ�֮���һ��ʢNaHCO3��Һ��ϴ��ƿ����ȥCO2�л��еĴ����������ʴ�Ϊ����ͬѧ˵���е�����Ӧ����ƿ���Թ�֮���һ��ʢNaHCO3��Һ��ϴ��ƿ����ȥCO2�л��еĴ���������

����Ŀ����ҵ����﮻�ʯΪԭ������̼��﮵IJ��ֹ�ҵ�������£�
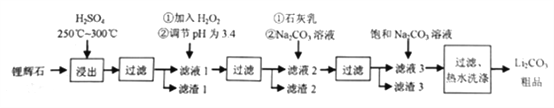
��֪����﮻�ʯ����Ҫ�ɷ�ΪLi2O��Al2O3��4SiO2�����к�����FeO��MgO�ȡ�
��Li2O��Al2O3��4SiO2+H2SO4![]() Li2SO4+Al2O3��4SiO2��H2O
Li2SO4+Al2O3��4SiO2��H2O
��ijЩ���ʵ��ܽ��(S)���±���ʾ��
T�� | 20 | 40 | 60 | 80 |
S(Li2CO3)/g | 1.33 | 1.17 | 1.01 | 0.85 |
S(Li2SO4)/g | 34.2 | 32.8 | 31.9 | 30.7 |
��Fe3+��ȫ����ʱpHΪ3.4
(1)Ϊ���ԭ�Ͻ������ʣ��������¶���ɲ�ȡ�Ĵ�ʩ��__________(��дһ��)��
(2)����Һ1�м���H2O2��Ŀ����__________(�����ӷ���ʽ��ʾ)������pH����Լ���__________(����� )��
A��CuO B��CuCO3 C��MgO D��NH3��H2O
(3)�������з����Al2O3����������ͼ��ʾ����д�����ɳ��������ӷ���ʽ__________��
![]()
(4)����Һ2�м���Na2CO3��Һ��������__________��
(5)�����ˡ���ˮϴ�ӡ������У�����ˮϴ�ӡ���ԭ����__________;֤��������ϴ���IJ�����__________��
