��Ŀ����
����Ŀ��Ŀǰ�����Ϻ�ˮ��������Ҫ���������������������������ȡ�������������ֱ����Դ������ͨ�����ӽ���Ĥ�Ժ�ˮ���д���(ԭ����ͼ��ʾ)��������������ѹǿ��ʹ��ˮһ���ˮ����ͨ����Ĥ���뵭ˮһ�࣬�Ӷ��õ���ˮ��Ũ��������Һ(ԭ����ͼ��ʾ)������˵����ȷ����
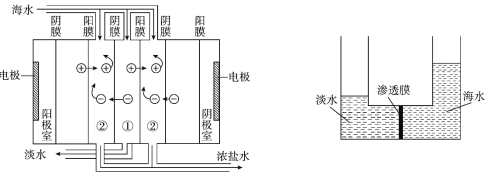
A. ������������������������������ѧ��Ӧ
B. ������������Ĥ����ֱ����Χ��1��100nm
C. �������豸���ɱ��͵��ŵ�
D. Ũ��������Һ��������ȡ���Ʊ�ʳ�Ρ�þ���������
���𰸡�D
��������
A.���������ᷢ���绯ѧ��Ӧ��A����
B.��Ĥֻ����ˮ���������������Ӳ������������ֱ��ӦС�ڽ�������ֱ������С��1nm��B����
C.�����豸�������һ����Ĵ�����Դ���ɱ��ϸߣ�C����
D.���Ũ���������Һ���д����Ȼ��ơ��Ȼ�þ��±�����������ȡ���Ʊ�ʳ�Ρ�þ��������ʣ���D��ȷ��
��ѡD��

����Ŀ����˾ƥ�֣�����ˮ���ᣬ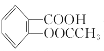 ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128����135����ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[��CH3CO��2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣���Ӧԭ�����£�
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128����135����ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[��CH3CO��2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣���Ӧԭ�����£�
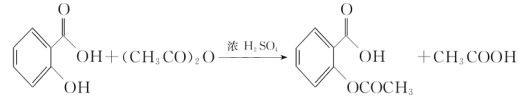
�Ʊ����������������£�
![]()
��Ҫ�Լ��Ͳ�Ʒ�������������±���ʾ��
���� | ��Է������� | �۵��е㣨�棩 | ˮ |
ˮ���� | 138 | 158���۵㣩 | �� |
������ | 102 | 139.4���е㣩 | ��ˮ�� |
����ˮ���� | 180 | 135���۵㣩 | �� |
�����������Ϣ�ش��������⣺
��1���Ʊ���˾ƥ��ʱ��Ҫʹ�ø����������ԭ����_____��
��2���ϳɰ�˾ƥ��ʱ������ʵļ��ȷ�����_____��
��3���ᴿ�ֲ�Ʒ�������£����Ȼ���װ����ͼ��
![]()
��ʹ���¶ȼƵ�Ŀ���ǿ��Ƽ��ȵ��¶ȣ���ֹ_____��
������ˮ������������_____������a������b������
�����ȹ��˵�ԭ����_____��
������˵����ȷ����_____����ѡ����ĸ����
a�������ᴿ�������������������������ܼ�
b�������ᴿ�ֲ�Ʒ�ķ������ؽᾧ
c�����������ᴿ���̿��Եó���˾ƥ�������������е��ܽ�ȵ���ʱ��
d����������ɫʯ����Һ�жϲ�Ʒ���Ƿ���δ��Ӧ���ˮ����

��4����ʵ����ԭ��������2.0gˮ���ᡢ5.0mL������������1.08g/cm3�������ճƵò�Ʒ����Ϊ2.2g������������ˮ����IJ���Ϊ_____���ðٷ�����ʾ��С�����һλ����
����Ŀ��PCl3���ij����Ȼ�������ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
�۵�/�� | �е�/�� | �ܶ�/ g��mL��1 | ���� | |
���� | 44.1 | 280.5 | 1.82 | 2P��3Cl2(����) |
PCl3 | ��112 | 75.5 | 1.574 | ��ˮ����H3PO3��HCl����O2����POCl3 |
(һ)�Ʊ�
��ͼ��ʵ�����Ʊ�PCl3��װ��(����������ʡ��)��

(1)�����ҵ�������________�����У�������ˮ��ˮ�����ӵĽӿڱ����________��(����a����b��)
(2)ʵ�����Ʊ�Cl2�����ӷ���ʽ___________________________��ʵ������У�Ϊ����PCl5�����ɣ�Ӧ����____________��
(3)��ʯ�ҵ����ã�һ�Ƿ�ֹ�����е�ˮ���������ʹPCl3ˮ�⣬Ӱ���Ʒ�Ĵ��ȣ�����_________��
(4)����������ͨ�����Cl2֮ǰ��Ӧ��ͨ��һ��ʱ��CO2�ž�װ���еĿ�������Ŀ����________��
(��)����
�ⶨ��Ʒ��PCl3���ȵķ������£�Ѹ�ٳ�ȡ4.100 g��Ʒ��ˮ����ȫ�����500 mL��Һ��ȡ��25.00 mL���������0.100 0 mol��L��1 20.00 mL����Һ����ַ�Ӧ������0.100 0 mol��L��1 Na2S2O3��Һ�ζ������ĵ⣬�յ�ʱ����12.00 mL Na2S2O3��Һ��
��֪��H3PO3��H2O��I2===H3PO4��2HI��I2��2Na2S2O3===2NaI��Na2S4O6������ⶨ������û��������Ӧ��
(5)�����������ݣ��ò�Ʒ��PCl3(��Է�������Ϊ137.5)����������Ϊ________�����ζ��յ�ʱ���Ӷ�������PCl3����������________(����ƫ������ƫС��������Ӱ����)��
(��)̽��
(6)���ʵ��֤��PCl3���л�ԭ�ԣ�_____________________________________��(��ѡ�Լ��У�����ˮ��ϡ���ᡢ��ˮ������)


