ЬтФПФкШн
ФГаЫШЄаЁзщОЪЕбщВтЖЈЃЌдк1ЁС105 Pa,298 KЬѕМўЯТЃЌ1 molЧтЦјЭъШЋШМЩеЩњГЩЫЎеєЦјЗХГі242 kJШШСПЃЌЯТСаШШЛЏбЇЗНГЬЪНе§ШЗЕФЪЧ(ЁЁЁЁ)
AЃЎH2O(g)=H2(g)ЃЋ O2(g)ЁЁІЄHЃНЃЋ242 kJЁЄmolЃ1
O2(g)ЁЁІЄHЃНЃЋ242 kJЁЄmolЃ1
BЃЎ2H2(g)ЃЋO2(g)=2H2O(l)ЁЁІЄHЃНЃ484 kJЁЄmolЃ1
CЃЎH2(g)ЃЋ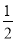 O2(g)=H2O(g)ЁЁІЄHЃНЃЋ242 kJЁЄmolЃ1
O2(g)=H2O(g)ЁЁІЄHЃНЃЋ242 kJЁЄmolЃ1
DЃЎ2H2(g)ЃЋO2(g)=2H2O(g)ЁЁІЄHЃНЃЋ484 kJЁЄmolЃ1
A
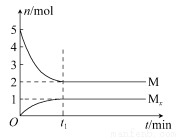
ЁОНтЮіЁП1 mol H2ЭъШЋШМЩеЩњГЩЫЎеєЦјЪБЗХГіЕФШШСПЮЊ242 kJЃЌЙЪCЁЂDбЁЯюжаШШСПжЕгІЮЊИКжЕЃЌВЛе§ШЗЃЛBбЁЯюH2OЕФзДЬЌгІЮЊЦјЬЌЃЌВЛе§ШЗЃЛH2OЕФЗжНтЗДгІгыЛЏКЯЗДгІЗНЯђЯрЗДЃЌЮќШШгыЗХШШЕФЪ§жЕЯрЕШЁЃ

СЗЯАВсЯЕСаД№АИ
ЯрЙиЬтФП