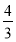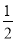��Ŀ����
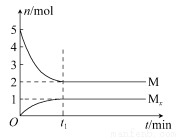
E�Ƿǽ�������ǿ��Ԫ�أ�M��E����̬�⻯��ڹ̶�������ܱ������У�����M�������¹�ϵ��xM(g) Mx(g)����Ӧ�������������ʵ�����ʱ��ı仯��ϵ��ͼ������˵����ȷ����(����)
Mx(g)����Ӧ�������������ʵ�����ʱ��ı仯��ϵ��ͼ������˵����ȷ����(����)
A���÷�Ӧ�Ļ�ѧ����ʽ��2HF (HF)2
(HF)2
B��ƽ��ʱ��������ƽ��Ħ��������33.3
C��t1ʱ�̣������¶Ȳ��䣬�ٳ���1 mol M�����´ﵽƽ��ʱ�� ������
������
D��M�ķе��ͬ������һ����Ԫ�ص���̬�⻯��е��
C
����������ͼ��֪��Ӧ���������Ļ�ѧ������֮��Ϊ3��1�����仯ѧ����ʽΪ3HF (HF)3��A����ƽ���������к�2 mol HF��1 mol (HF)3��
(HF)3��A����ƽ���������к�2 mol HF��1 mol (HF)3��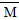 ��(2 mol��20 g��mol��1��1 mol��60g��mol��1)��3 mol��33.3 g��mol��1��ѡ��B��©д��λ������ѡ��C�൱�ڶ���ϵʵʩ��ѹ������ѹǿ�����ڷ�Ӧ������У��ʱ�ֵ����C��ȷ������HF���Ӽ����������γɵϷ��ӣ�����е��HCl�ߣ�D����
��(2 mol��20 g��mol��1��1 mol��60g��mol��1)��3 mol��33.3 g��mol��1��ѡ��B��©д��λ������ѡ��C�൱�ڶ���ϵʵʩ��ѹ������ѹǿ�����ڷ�Ӧ������У��ʱ�ֵ����C��ȷ������HF���Ӽ����������γɵϷ��ӣ�����е��HCl�ߣ�D����

��ϰ��ϵ�д�
�����Ŀ