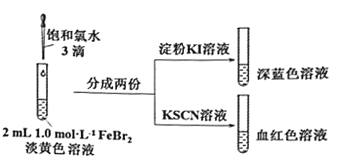
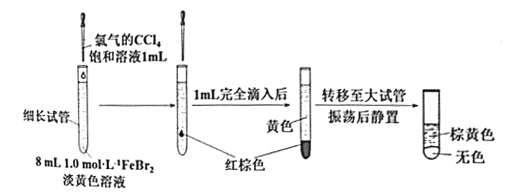
��Ŀ����
����Ŀ��ij����С��������CuO��NH3��Ӧ���о�NH3��ij�����ʲ��ⶨ����ɣ����������ʵ��װ�ã��г�װ��δ����������ʵ�顣��ش��������⣺
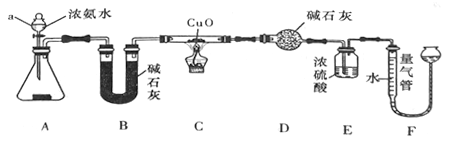
��1������a������Ϊ______________��
��2��ʵ���й۲쵽װ��C�к�ɫCuO��ĩ��Ϊ��ɫ���壬������������ɫ��ζ�������������������֤��NH3 ����______�ԣ�д����Ӧ�Ļ�ѧ����ʽ��______________��
��3��Eװ����Ũ�����������______________��
��4��ʵ����ϣ�����ø����D����mg��װ��F�����������ΪVL��������ɱ�״����,�����е������ԭ�Ӹ�����ֵΪ_____���ú�m��V��ĸ�Ĵ���ʽ��ʾ����
���𰸡� ��Һ©�� ��ԭ 3CuO+2NH3![]() 3Cu+3H2O+N2 ����δ��Ӧ�İ�������ֹF��ˮ������������D
3Cu+3H2O+N2 ����δ��Ӧ�İ�������ֹF��ˮ������������D ![]()
��������CuO��NH3��Ӧ����H2O��������Cu��C�к�ɫ������ɫ���������ֳ���ԭ�ԣ�Ũ�������ղ�����ˮ��������ͨ��ˮ���������Լ����HԪ�ص�������Fװ��ͨ����ˮ������������N2�������ת��Ϊ�����������NԪ�ص��������Ӷ��������N��H ԭ�Ӹ����ȣ���һ�������������ɡ�
��1��a����Ϊ����Һ©��
��2����ʵ�������֪CuO��NH3��Ӧ������Cu���ʺ͵�����CuO�����˻�ԭ��Ӧ���ʰ��������˻�ԭ�ԡ������ķ�ӦΪ��3CuO+2NH3![]() N2+3H2O+3Cu
N2+3H2O+3Cu
��3����Ũ���������������δ��CuO��ȫ��Ӧ�İ�������ֹ��������Fװ������������ͬʱҲ��ֹFװ���е�ˮ������������D������ˮ������ƫ������������
��4�������D ���ӵ�����ΪH2O������Ϊmg����ˮ�����ʵ���Ϊ��m/18��mol��Hԭ�ӵ����ʵ���Ϊ��m/9��mol��N2�����ʵ���Ϊ��V/22.4��mol����ԭ�ӵ����ʵ���Ϊ��V/11.2��mol���������е������ԭ�Ӹ�����ֵΪ(V/11.2)��(m/9)=9v/11.2m