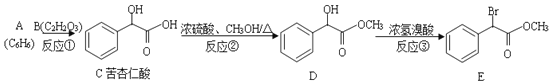
��Ŀ����
����Ŀ��������һ����Ҫ�Ļ���ԭ�ϡ�
��1��������ʯ���鷴Ӧ�����Ƶ�Ư�ۣ���Ư�۱�¶�ڿ������������˿����е�CO2��H2O�����ܲ��ֱ��ʣ�д����֤Ư���Ѳ��ֱ��ʵ�ʵ�鷽����________��
��2����ˮ�к��ж��ֳɷ֣�������ж������ʣ�����������ˮ�ֱ�����ͼ�������ʷ����ķ�Ӧ��գ�a��b��c��d���غϲ��ִ������ʼ䷴Ӧ������ˮ������
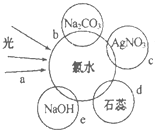
c���̵����ӷ���ʽΪ_______��
e������������ԭ��Ӧ�Ļ�ѧ����ʽΪ________��
d�������۲쵽������Ϊ_______��
b����֤������ˮ�д���_________������ĸ������
A Cl2 B Cl- C HClO DH+
��3�������±�������ĵ��볣����д�����ֿ������ӱ�����ˮ�еĴ������Ũ�ȵ�����_______��˵��ѡ������ɻ�������_________��
ijЩ����ĵ��볣����298K�� | ||
��Ԫ���� | K1 | K2 |
HClO | 2.95��10-8 | |
CH3COOH | 1.76��10-5 | |
H2SO3 | 1.54��10-2 | 1.02��10-17 |
H2CO3 | 4.30��10-7 | 5.61��10-11 |
���𰸡�ȡ�����ò�����պȡ����Ư����Һ����ʯ����ֽ�ϣ�ʯ����ֽ��ɫ����ȡ�����������ᣬ�����ݲ�����˵��Ư���Ѳ��ֱ��� Ag++Cl-=AgCl�� Cl2+2NaOH=NaCl+NaClO+H2O ʯ���ȱ�����ɫ D NaHCO3��CH3COONa ���������ֻ�������ᷴӦ������������ᷴӦ����ʹ��ӦCl2+H2O![]() HCl+HClO�����ƶ�
HCl+HClO�����ƶ�
��������
��1��Ư�۱�¶�ڿ������������˿����е�CO2��H2O�����ܲ��ֱ��ʣ��˹��̷����ķ�ӦΪ��Ca(ClO)2+CO2+H2O=2HClO+CaCO3����Ҫ����֤Ư���Ѳ��ֱ��ʣ�ֻ����֤�˱��ʷ�Ӧ�IJ��T�ɡ�HClO��Ư���ԣ��ݴ�ȡ�����ò�����պȡ����Ư����Һ����ʯ����ֽ�ϣ�ʯ����ֽ��ɫ��CaCO3���Ժ����ᷴӦ���ɶ�����̼���ݴ���ȡ�����������ᣬ�����ݲ�����������������֤Ư���Ѳ��ֱ��ʵ�ʵ�鷽��Ϊ��ȡ�����ò�����պȡ����Ư����Һ����ʯ����ֽ�ϣ�ʯ����ֽ��ɫ����ȡ�����������ᣬ�����ݲ�����˵��Ư���Ѳ��ֱ��ʡ�
��Ϊ��ȡ�����ò�����պȡ����Ư����Һ����ʯ����ֽ�ϣ�ʯ����ֽ��ɫ����ȡ�����������ᣬ�����ݲ�����˵��Ư���Ѳ��ֱ��ʡ�
��2��������ˮ�ijɷ��У�Cl2��HClO��H2O��H+��Cl-��ClO-��OH-(����)��c��������ˮ�е�Cl-��Ag+��Ӧ�Ĺ��̣����ӷ���ʽΪ��Ag++Cl-=AgCl����e��������ˮ�е�Cl2��NaOH
��Ӧ����ѧ����ʽΪ��Cl2+2NaOH=NaCl+NaClO+H2O��������ˮ�к�������ʹ����ᣬ������ǿ�ᣬ����ʹ��ɫʯ���죬��������Ư���ԣ�����d�������۲쵽������Ϊ���ȱ�����ɫ��b��������ˮ�е������̼���Ʒ�Ӧ���ɶ�����̼��֤����ˮ����H+����ѡD��
��Ϊ��Ag++Cl-=AgCl����Cl2+2NaOH=NaCl+NaClO+H2O��ʯ���ȱ�����ɫ��D��
��3����ˮ�д�������Դ�ڣ�Cl2+H2O![]() HCl+HClO��Ҫ�����ӱ�����ˮ�еĴ������Ũ�ȣ���ʹ�÷�Ӧƽ�������ƶ�������ͨ����������ķ���ʵ�֡������ӱ�����ˮ�еĴ�����Ũ�ȵ����Σ�Ӧ���㣺�����ᷴӦ���ֲ���HClO��Ӧ�����ݱ����и����ĵ��볣����ֵ�����Եó�����ǿ��Ϊ��CH3COOH> H2CO3> HClO > HCO3-�����Գ����ķ������������ο�����NaHCO3��CH3COONa��
HCl+HClO��Ҫ�����ӱ�����ˮ�еĴ������Ũ�ȣ���ʹ�÷�Ӧƽ�������ƶ�������ͨ����������ķ���ʵ�֡������ӱ�����ˮ�еĴ�����Ũ�ȵ����Σ�Ӧ���㣺�����ᷴӦ���ֲ���HClO��Ӧ�����ݱ����и����ĵ��볣����ֵ�����Եó�����ǿ��Ϊ��CH3COOH> H2CO3> HClO > HCO3-�����Գ����ķ������������ο�����NaHCO3��CH3COONa��
��Ϊ��NaHCO3��CH3COONa�����������ֻ�������ᷴӦ������������ᷴӦ����ʹ��ӦCl2+H2O![]() HCl+HClO�����ƶ���
HCl+HClO�����ƶ���

����Ŀ�������ᣨ![]() ������Ҫ�Ļ���ԭ�ϣ���Ӧ��������������Ⱦ�����塢���ܼ������ϼ�ʳƷ��������������Ҳ�����ڸ����豸�ķ������ij��ѧʵ��С����ʵ�������Ա���ȩΪԭ����ȡ���������Ʒ���״���
������Ҫ�Ļ���ԭ�ϣ���Ӧ��������������Ⱦ�����塢���ܼ������ϼ�ʳƷ��������������Ҳ�����ڸ����豸�ķ������ij��ѧʵ��С����ʵ�������Ա���ȩΪԭ����ȡ���������Ʒ���״���![]() ����ʵ������:
����ʵ������:
��֪���� ��
��![]() ��
�� ��R��R1��ʾ��������ԭ�ӣ�
��R��R1��ʾ��������ԭ�ӣ�
��������ʵIJ����������ʼ�����
���� | ����ܶ� | �۵�/�� | �е�/�� | �ܽ�� | |
ˮ | ���� | ||||
����ȩ | 1.04 | ��26 | 179.6 | �� | ���� |
������ | 1.27 | 122.1 | 249 | 25���ܣ�95������ | ���� |
���״� | 1.04 | ��15.3 | 205.7 | �� | ���� |
���� | 0.71 | ��116.3 | 34.6 | ���� | �� |
��ش���������:
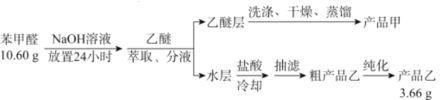
��1��������ȡ����Һ����ʱ���ò�������������Ϊ___________����Һʱ�����Ѳ�Ӧ��_______(�����¿ڷų��������Ͽڵ�����)��
��2��ϴ�����Ѳ�ʱ��Ҫ������NaHSO3��Һ��10%Na2CO3��Һ������ˮ����ϴ�ӡ����м���NaHSO3��Һϴ�ӵ���ҪĿ����________________����Ӧ�Ļ�ѧ����ʽΪ___________________________��
��3�������ò�Ʒ��ʱ�������Ƭ��Ŀ��Ϊ_____________������ʱӦ�����¶���____�����ҡ�
A.34.6 B.179.6 C.205.7 D.249
��4���ᴿ�ֲ�Ʒ�һ�ò�Ʒ�ҵĴ�����������Ϊ________________��
��5����ȡ10.60g�ı���ȩ����ʵ�飬������ȡ��Ʒ�ҵ�����Ϊ3.66g�����Ʒ�ҵIJ���Ϊ____________��
����Ŀ����ʽ������(NiOOH)�����������ص��������ϣ����÷�����������Ҫ��Ni��Al������Cr��FeS ��)���Ʊ����乤���������£�

�ش��������⣺
(1)�����ݳ�����ʱ��������Ӧ�����ӷ�Ӧ����ʽΪ_________________________;
(2)���ܽ⡱ʱ�ų�������Ϊ_______________ (�ѧʽ);
(3)��֪�������½������ӿ�ʼ��������ȫ������pH���±���
��ʼ������pH | ��ȫ������pH | |
Ni2+ | 6.2 | 8.6 |
Fe2+ | 7.6 | 9.1 |
Fe3+ | 2.3 | 3.3 |
Cr3+ | 4.5 | 5.6 |
����pH 1��ʱ����ҺpH��ΧΪ______________________��
(4)�ڿ����м���Ni(OH)2�ɵ�NiOOH,��д���˷�Ӧ�Ļ�ѧ����ʽ_____________;

(5)����������Һ���ж��ִ�����ʽ, CrO42����Cr2O72������Һ�п��ת���������£���ʼŨ��Ϊ1.0mol/L��Na2CrO4��Һ��c(Cr2O72��)��c(H+)�ı仯��ͼ��ʾ�������ӷ���ʽ��ʾNa2CrO4��Һ�е�ת����Ӧ________________������A�����ݼ������ת����Ӧ��ƽ�ⳣ��Ϊ______________,�¶����ߣ���Һ��CrO42����ƽ��ת���ʼ�С����÷�Ӧ�ġ�H____0���>������<����=������