��Ŀ����
����Ŀ���⻯���ƣ�NaAlH4����һ���������ʴ�����ϣ���������Ti��NaAlH4��150��ʱ���⣬��170����15.2MPa���������ظ����⡣NaAlH4����AlCl3��NaH���ʵ������ºϳɣ�NaAlH4�ľ����ṹ��ͼ��ʾ��
��1����̬Tiԭ�ӵļ۵��ӹ����ʾʽΪ ��
��2��NaH���۵�Ϊ800�����������л��ܼ���NaH���� ���壬�����ʽΪ ��
��3��AlH4-�У�Al�Ĺ���ӻ���ʽΪ ��������AlH4-�ռ乹����ͬ��һ�����Ӻ�һ�ַ��� �� ���ѧʽ����
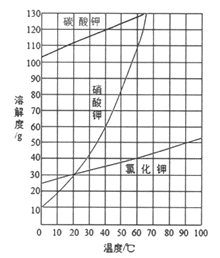
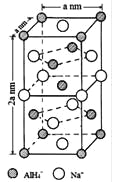
��4��NaAlH4�����У���AlH4-�����ҵȾ��Na+�� ����NaAlH4������ܶ�Ϊ gcm-3���ú�a�Ĵ���ʽ��ʾ������NaAlH4�������Ĵ���Na+��Li+ȡ�����õ��ľ���Ϊ ���ѧʽ����
��5��AlCl3��178��ʱ����������������Է�������ԼΪ267����������������λ�����������ӵĽṹʽΪ ��������λ������
��6��NaAlH4���������Ϊ��ÿ3��AlH4-�У���2���ֱ��ͷų�3��Hԭ�Ӻ�1��Alԭ�ӣ�ͬʱ���Alԭ������ڵ�Naԭ��ת�Ƶ����ͷŵ�Alԭ�����µĿ�λ���γ��µĽṹ�����ֽṹ�仯�ɱ������չ���������壬�Ӷ��ͷų���������������̿��û�ѧ����ʽ��ʾΪ ��
���𰸡���1��![]()
��2������ Na+[:H]-
��3��sp3�ӻ� NH4����CH4
��4��8![]() Na3Li��AlH4��4
Na3Li��AlH4��4
��5��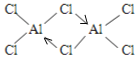
��6��3NaAlH4�TNa3AlH6+2Al+3H2��
�������������������1��Ti��ԭ������Ϊ22�������Ų�Ϊ1s22s22p63s23p63d24s2���۵����Ų�ʽΪ3d24s2���۵��ӹ����ʾʽΪ![]() ��
��
��2��NaH���۵�Ϊ800�����������л��ܼ����������Ӿ��壻NaH�����ӻ��������ʽΪNa+[:H]-��
��3��AlH4-�У�Al�ļ۲���Ӷ���=4+1/2��3+1-1��4��=4������Alԭ��Ϊsp3�ӻ����ȵ�����Ľṹ���ƣ�������AlH4-�ռ乹����ͬ��������NH4+��BH4-��SO42-��PO43-�ȣ�������CH4��CCl4�ȡ�
��4���Ե���Na+�����о�����֮�����AlH4-����Ϊ![]() anm��λ��ͬһ���4�������Լ�����4������������ϣ�����8�������ݾ�̯����֪�������������ӵĸ���Ϊ6��1/2+4��1/4������Ļ�ѧʽΪNaAlH4�����Ծ�����AlH4-�ĸ���Ϊ4���þ���������Ϊ(54��4)��NAg���þ������Ϊ2a3nm3=2a3��10-21cm3����þ������ܶ�Ϊ
anm��λ��ͬһ���4�������Լ�����4������������ϣ�����8�������ݾ�̯����֪�������������ӵĸ���Ϊ6��1/2+4��1/4������Ļ�ѧʽΪNaAlH4�����Ծ�����AlH4-�ĸ���Ϊ4���þ���������Ϊ(54��4)��NAg���þ������Ϊ2a3nm3=2a3��10-21cm3����þ������ܶ�Ϊ![]() g��cm-3=
g��cm-3=![]() g��cm-3��������AlH4-�ĸ���Ϊ4�������ӵĸ���Ϊ4����NaAlH4�������Ĵ���Na+��Li+ȡ��������AlH4-�ĸ���Ϊ4�������ӵĸ���Ϊ3������ӵĸ���Ϊ1������Ļ�ѧʽΪ��Na3Li��AlH4��4��
g��cm-3��������AlH4-�ĸ���Ϊ4�������ӵĸ���Ϊ4����NaAlH4�������Ĵ���Na+��Li+ȡ��������AlH4-�ĸ���Ϊ4�������ӵĸ���Ϊ3������ӵĸ���Ϊ1������Ļ�ѧʽΪ��Na3Li��AlH4��4��
��5���Ȼ�������ԭ����������ֻ��3�����ӣ��γ�3�����ۼ���ÿ����ԭ�Ӻ��ĸ���ԭ���γɹ��ۼ���������һ�����õ��Ӷ�����ԭ���ṩ�γɵ���λ������ͼ![]() ��
��
��6��NaAlH4���������Ϊ��ÿ3��AlH4-�У���2���ֱ��ͷų�3��Hԭ�Ӻ�1��Alԭ�ӣ�ͬʱ���Alԭ������ڵ�Naԭ��ת�Ƶ����ͷŵ�Alԭ�����µĿ�λ����������Al��H2��AlH63-����Ӧ�ķ���ʽΪ��3NaAlH4�TNa3AlH6+2Al+3H2����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ͼ��ʾװ�ý�������ʵ�飺��������Һ������У�Ԥ���������ʵ���������
ѡ�� | �������� | �������� | Ԥ����е����� |
A | ϡ���� | ̼�������������ƵĻ����Һ | ������������ |
B | Ũ���� | ��ɰֽ��ĥ�������� | ��������ɫ���� |
C | �Ȼ�����Һ | Ũ����������Һ | ����������ɫ���� |
D | ������Һ | ���Ը��������Һ | ��Һ����ɫ |
A. A B. B C. C D. D