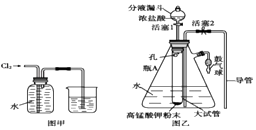
��Ŀ����
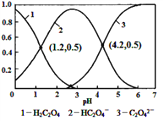
����Ŀ�������£�0.1 mol/L��H2C2O4��Һ��H2C2O4��HC2O4-��C2O42-��������ռ���ʵ����������ֲ�ϵ������pH�仯�Ĺ�ϵ����ͼ��ʾ�����б�������ȷ����
A. HC2O4-![]() H+��C2O42-��K��1��10-4.2
H+��C2O42-��K��1��10-4.2
B. �������ʵ�����NaHC2O4��Na2C2O4����ˮ�У�������ҺpHǡ��Ϊ4.2
C. ������HF��K��1��10-3.4��������H2C2O4��Һ���뵽����NaF��Һ�У������ķ�ӦΪ��H2C2O4��F-��HF��HC2O4-
D. ��0.1 mol/L NaHC2O4��Һ�У�������Ũ�ȴ�С��ϵΪ��c(Na+)��c(HC2O4-)��c(H+)��c(C2O42-)��c(OH-)
���𰸡�B
������������ͼ����PH=4.2ʱ��c(HC2O4-)=c(C2O42-)�� HC2O4-![]() H+��C2O42-��K��
H+��C2O42-��K��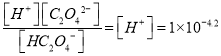 ����A��ȷ��HC2O4-�ĵ���ƽ�ⳣ����1��10-4.2�� C2O42-��ˮ��ƽ�ⳣ����
����A��ȷ��HC2O4-�ĵ���ƽ�ⳣ����1��10-4.2�� C2O42-��ˮ��ƽ�ⳣ����![]() ��HC2O4-�ĵ������C2O42-��ˮ�������Խ������ʵ�����NaHC2O4��Na2C2O4����ˮ��c(HC2O4-)��c(C2O42-)����pH��4.2����B����������ͼ����PH=4.2ʱ��c(HC2O4-)=c(C2O42-)����H2C2O4��
��HC2O4-�ĵ������C2O42-��ˮ�������Խ������ʵ�����NaHC2O4��Na2C2O4����ˮ��c(HC2O4-)��c(C2O42-)����pH��4.2����B����������ͼ����PH=4.2ʱ��c(HC2O4-)=c(C2O42-)����H2C2O4��![]() ��PH=1.2ʱ��c(HC2O4-)=c(H2C2O4)��H2C2O4��
��PH=1.2ʱ��c(HC2O4-)=c(H2C2O4)��H2C2O4��![]() ��
�� ![]() ��������H2C2O4��Һ���뵽����NaF��Һ�У������ķ�ӦΪ��H2C2O4��F-��HF��HC2O4-����C��ȷ��NaHC2O4��Һ��HC2O4-�ĵ���ƽ�ⳣ����1��10-4.2��HC2O4-��ˮ��ƽ�ⳣ����
��������H2C2O4��Һ���뵽����NaF��Һ�У������ķ�ӦΪ��H2C2O4��F-��HF��HC2O4-����C��ȷ��NaHC2O4��Һ��HC2O4-�ĵ���ƽ�ⳣ����1��10-4.2��HC2O4-��ˮ��ƽ�ⳣ����![]() ��HC2O4-�������ˮ�⣬����0.1 mol/L NaHC2O4��Һ�����ԣ�������Ũ�ȴ�С��ϵΪ��c(Na+)��c(HC2O4-)��c(H+)��c(C2O42-)��c(OH-)����D��ȷ��
��HC2O4-�������ˮ�⣬����0.1 mol/L NaHC2O4��Һ�����ԣ�������Ũ�ȴ�С��ϵΪ��c(Na+)��c(HC2O4-)��c(H+)��c(C2O42-)��c(OH-)����D��ȷ��

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�����Ŀ�������ѣ�Ti������Ϊ21���ͽ������䵥�ʺͻ�������й㷺��Ӧ�ü�ֵ����ش��������⣺
��1��Ti�Ļ�̬ԭ�Ӽ۵����Ų�ʽΪ______��
��2������TiO2������������Ӧ�Ĵ�����

������ķ����в�ȡsp2��ʽ�ӻ���̼ԭ����_____�������������в�ȡsp3��ʽ�ӻ���ԭ�Ӷ�ӦԪ�صĵ縺���ɴ�С��˳��Ϊ_____��
��3��ij��Ti3+�����Ļ�ѧʽΪ[TiCl(H2O)5]Cl2��H2O��������������к��еĻ�ѧ��������_____��1 mol��������к��е�������Ŀ��_____��
��4��ͨ��x������֪̽KCl��MgO��CaO��TiN�ľ�����NaCl�ľ���ṹ���ƣ���֪�������Ӿ���ľ������������£�
���Ӿ��� | NaCl | KCl | CaO |
������/kJ��mol��1 | 786 | 715 | 3401 |
KCl��MgO��CaO��TiN�������Ӿ����۵��ɸߵ��͵�˳��Ϊ_____��
��5��������������ͬ�������壬�������������ѻ��������������������ѻ�����ͼ��ʾ���Ѿ����һ�־�������������a��0.469 nm��c��0.295 nm������Ѿ�����ܶ�Ϊ______g��cm-3����NA ��ʾ�����ӵ�������ֵ���г�����ʽ���ɣ���