��Ŀ����
����Ŀ����������һ�������Դ����ˮú����ȡ�����ѵ�ԭ�����£�
I. CO(g)+2H2(g)![]() CH3OH(g)
CH3OH(g)
II. 2CH3OH(g)===CH3OCH3(g)+H2O(g)
��1��300����500��ʱ����ӦI��ƽ�ⳣ���ֱ�ΪK1��K2����K1��K2����������ӦΪ______��Ӧ����������������������������
��2���ں����ܱ������з�����ӦI:
����ͼ����ȷ��ӳ��ϵ�м״�����������¶ȱ仯�����������____������a������b������
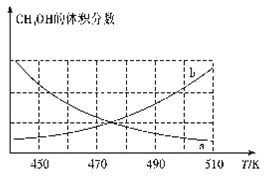
������˵���ܱ�����Ӧ�Ѵ�ƽ��״̬����____�����ţ���
A.�����������ѹǿ���ٱ仯 B.���������ܶȲ��ٱ仯
C����������ƽ����Է����������ٱ仯 D��v��(H2)=2v��(CH3OH)
��3��500Kʱ����2L�ܱ������г���4molCO��8molH2��4min�ﵽƽ�⣬ƽ��ʱCO��ת����Ϊ80%����2c(CH3OH)=c(CH3OCH3) ����
��0��4min����ӦI��v(H2)=______����ӦI��ƽ�ⳣ��K=______��
�ڷ�ӦII��CH3OH��ת������=_______��
���𰸡� ���� a AC 0.8 mol��L-1��min-1 1.25 80%
����������1��300����500��ʱ����ӦI��ƽ�ⳣ���ֱ�ΪK1��K2����K1��K2���¶�������Kֵ��С����K�ı���ʽ��֪��ƽ�������ƶ���������ӦΪ���ȷ�Ӧ��
��2�����ں����ܱ������з�����ӦI: CO(g)+2H2(g)![]() CH3OH(g) ��H��0����ͼ�����������¶�ƽ�������ƶ���CH3OH�İٷֺ�����С����a���߷��ϡ�
CH3OH(g) ��H��0����ͼ�����������¶�ƽ�������ƶ���CH3OH�İٷֺ�����С����a���߷��ϡ�
��A. �淴Ӧ���У����������ܵ����ʵ�����С��ѹǿ�DZ仯��������Ӧ�����������ѹǿ���ٱ仯��˵����Ӧ�ﵽƽ�⣬A��ȷ�� B. ��Ϊ���壬������������䣬�����������������䣬�ܶ���ʼ����Ϊ��ֵ���ܶ��Dz�������B������ C�������������������䣬�淴Ӧ���У����������ܵ����ʵ�����С����ƽ����Է���������С�����Ϊ��ֵ��˵����Ӧ�ﵽƽ�⣬C��ȷ��D��v��(H2)��v��(CH3OH) ������ͬ������˵���ﵽƽ���� ѡAC��
��3��ij�¶��£���4molCO��8molH2����2L���ܱ������У�4min�ﵽƽ�⣬ƽ��ʱCO��ת����Ϊ80%����2c(CH3OH)=c(CH3OCH3)
���ڿ��淴Ӧ��CO��g��+2H2��g��CH3OH��g�� 2CH3OH(g)===CH3OCH3(g)+H2O(g)
��ʼ��mol/L����2 4 0 1.6 0 0
�仯��mol/L����1.6 3.2 1.6 2x x
ƽ�⣨mol/L����0.4 0.8 1.6 1.6-2x x
(1.6-x)/x=1/2 x=0.64 ��ƽ��ʱc(CH3OH)= 1.6-2x=0.32 mol/L
��0��4min����ӦI��v(H2)=3.2 mol/L/4min= 0.8 mol��L-1��min-1����ӦI��ƽ�ⳣ��K=0.32/0.4��0.82=1.25��
�ڷ�ӦII��CH3OH��ת������=2x/1.6=0.64��2/1.6==80%

����Ŀ��I���״���һ����Ҫ����ԭ�ϣ�����һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ����
��1����֪��CH3OH(g)=HCHO(g)+H2(g) ��H=+84 kJ/mol 2H2(g)+O2(g)=2H2O(g) ��H=-484 kJ/mol����ҵ�ϳ��Լ״�Ϊԭ����ȡ��ȩ����д�� CH3OH (g)��O2 (g)��Ӧ����HCHO(g)��H2O(g)���Ȼ�ѧ����ʽ��_______________________��
��2���ɼ״���������NaOH��Һ���ɵ������ֻ���أ���ʹ�ֻ�����ʹ��һ���²ų�һ�ε硣�õ�ظ����ĵ缫��ӦʽΪ________________��
������2L�ܱ������У�800��ʱ��Ӧ2NO(g)+O2 (g)![]() 2NO2 (g)��ϵ�У�n(NO)��ʱ��ı仯�����
2NO2 (g)��ϵ�У�n(NO)��ʱ��ı仯�����
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)/mol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
��1����ͼ��ʾ����ʾNO2�仯���ߵ���_______����O2��ʾ��0��2 s�ڸ÷�Ӧ��ƽ������v=_______��
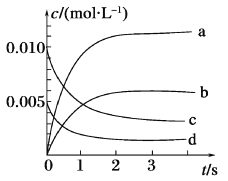
��2����˵���÷�Ӧ�Ѵﵽƽ��״̬����_______������ţ���
a��v(NO2) =2V(O2) b��������ѹǿ���ֲ���
c����ϵ��ɫ���ٸı� d���������ܶȱ��ֲ���

