��Ŀ����
����Ŀ�������ڱ���������ر���Һ���ⶨijNaOH��Һ��Ũ�ȣ��������²�����
����I������ƽ��ȷ��ȡ5.207g�ڱ���������أ���Է�������Ϊ204.2���������250mL����Һ��
�������ȡ25.00mL����Һ��250mL��ƿ�У�����1��2�η�̪��Һ����NaOH������Һ�ζ����յ㡣�ٰ����������ظ�����2�Ρ�
��1������I�У������ڱ���������ر���Һ�����ʵ���Ũ����__________________________��
��2���ζ������¼���£�
�ζ����� | ����Һ�����/mL | ������Һ����� | |
�ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
1 | 25.00 | 1.02 | 21.04 |
2 | 25.00 | 2.00 | 21.98 |
3 | 25.00 | 0.20 | 20.20 |
�ٵζ������з�����Ӧ�Ļ�ѧ����ʽ�ǣ��л���д�ṹ��ʽ��____________________��
�ڴ���NaOH��Һ�����ʵ���Ũ��Ϊ___________��
��3�����ݲ������գ�
�ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH������Һ���еζ�������NaOH��Һ��Ũ��_______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ƿ������ˮϴ�Ӻ�ˮδ��������ζ�ʱ��ȥNaOH������Һ�����______���ƫ����ƫС������Ӱ�족����
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�_______________��
�ܵζ��յ�ʱ��Һ����ɫ��α仯��____________________��
���𰸡� 0.1020mol��L 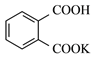 �� NaOH ����
�� NaOH ����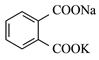 �� H2O 0.1275mol��L ƫ�� ��Ӱ�� ��ƿ����Һ��ɫ�ı仯 ����ɫ��Ϊdz��ɫ��������ڲ���ɫ
�� H2O 0.1275mol��L ƫ�� ��Ӱ�� ��ƿ����Һ��ɫ�ı仯 ����ɫ��Ϊdz��ɫ��������ڲ���ɫ
����������1���ڱ���������ص����ʵ�����5.207g��204.2g/mol��0.0255mol����Ũ����0.0255mol��0.25L��0.1020mol/L����2���ٵζ������з�����Ӧ�Ļ�ѧ����ʽ��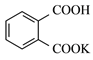 ��NaOH��
��NaOH�� ��H2O���ڸ��ݱ������ݿ�֪����������������ֱ���20.02mL��19.98mL��20.00mL�����ƽ��ֵ��20.00mL������ݷ���ʽ��֪����NaOH��Һ�����ʵ���Ũ��Ϊ
��H2O���ڸ��ݱ������ݿ�֪����������������ֱ���20.02mL��19.98mL��20.00mL�����ƽ��ֵ��20.00mL������ݷ���ʽ��֪����NaOH��Һ�����ʵ���Ũ��Ϊ![]() =0.1275mol/L����3���ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH������Һ���еζ�����������Ũ�ȼ�С����������������Һ������ӣ�����NaOH��Һ��Ũ��ƫ�͡�����ƿ������ˮϴ�Ӻ�ˮδ����������Ӱ�������۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯�����յ�ʱ��Һ�Լ��ԣ���ζ��յ�ʱ��Һ����ɫ�仯Ϊ����ɫ��Ϊdz��ɫ��������ڲ���ɫ��
=0.1275mol/L����3���ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH������Һ���еζ�����������Ũ�ȼ�С����������������Һ������ӣ�����NaOH��Һ��Ũ��ƫ�͡�����ƿ������ˮϴ�Ӻ�ˮδ����������Ӱ�������۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯�����յ�ʱ��Һ�Լ��ԣ���ζ��յ�ʱ��Һ����ɫ�仯Ϊ����ɫ��Ϊdz��ɫ��������ڲ���ɫ��

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�����Ŀ������A��E����ͬ��Ԫ�أ�������ѧ��ѧ�г��������ʣ�CΪ��ɫ������ˮ�Ĺ��壬���ǿɷ�������ͼ����ʾ�ķ�Ӧ(��A��E���������������ȥ)��

(1)д����Ӧ���ʵ����
���� | B | C | D |
��� | ___________ | ______________ | ______________ |
(2)�����Ϸ�Ӧ��(��������)��
����������ԭ��Ӧ����________�����ڸ��ֽⷴӦ����________��
(3)д����Ӧ�ۺ͢ߵ����ӷ���ʽ��_______________________��
����Ŀ��TiCl4���Ʊ��Ѽ��仯�������Ҫ�м��壬ijС��ͬѧ��������װ����ʵ�����Ʊ�TiCl4�����ʵ�����£��г�װ����ȥ����
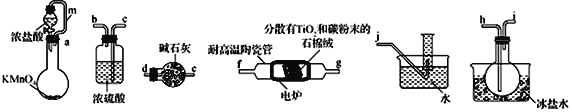
�����Ϣ���±���ʾ��
�۵�/�� | �е�/�� | �ܶ�/(g cm-3) | ˮ���� | |
TiCl4 | -25 | 136 | 1.5 | ��ˮ�⣬�������л��ܼ� |
CC14 | -23 | 76.8 | 1.6 | ������ˮ |
��ش��������⣺
��1���������������ҵķ�������װ�ú���������˳��Ϊ_____________���������ӿ���ĸ��
��2������������ʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ��_____________
������ȷ��˳���������в�������ĸ����
A.�رշ�Һ©������ B.ֹͣ���ȣ������ȴ
C.��Һ©������ D.����װ��D���մɹ�
ʵ��ʱ�����۲쵽______________ʱ����ʼ���в���D��
��3��װ��A�е���m������Ϊ_______________________��
��4��װ��C������Ϊ ___________________________________________��
��5��װ��D�г�����TiCl4�⣬ͬʱ����һ����̬������������÷�Ӧ�Ļ�ѧ����ʽΪ__________________________________��
��6�����ʵ��֤��װ�� F���ռ�����Һ���к���TiCl4��______________________________________________��
��7���Ƶõ�TiCl4�г���������CCl4���ӻ��Һ���з����TiCl4�IJ�������Ϊ_________________��





