��Ŀ����
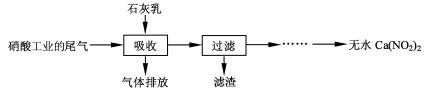
[2012�����վ�]��10�֣�����ʯ��������Ṥҵ��β��(��NO��NO2)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��Ca(NO2)2���䲿�ֹ����������£�
��1��һ�������£�NO��NO2�������з�Ӧ��NO(g)��NO2(g) N2O3(g)����ƽ�ⳣ������ʽΪK��________��
N2O3(g)����ƽ�ⳣ������ʽΪK��________��
��2�����������в�������Һ�����Ӵ�����(β�������������룬ʯ�����������������)����Ŀ����_____________ _________��������ѭ��ʹ�ã���������Ҫ�ɷ���__________(�ѧʽ)��
��3���ù��������NO��NO2���ʵ���֮�Ƚӽ�1��1����n(NO)��n(NO2)��1��1����ᵼ��______________________ ___����n(NO)��n(NO2)��1��1����ᵼ��_________________________________��
��4����������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽΪ______________________________��

��1��һ�������£�NO��NO2�������з�Ӧ��NO(g)��NO2(g)
 N2O3(g)����ƽ�ⳣ������ʽΪK��________��
N2O3(g)����ƽ�ⳣ������ʽΪK��________����2�����������в�������Һ�����Ӵ�����(β�������������룬ʯ�����������������)����Ŀ����_____________ _________��������ѭ��ʹ�ã���������Ҫ�ɷ���__________(�ѧʽ)��
��3���ù��������NO��NO2���ʵ���֮�Ƚӽ�1��1����n(NO)��n(NO2)��1��1����ᵼ��______________________ ___����n(NO)��n(NO2)��1��1����ᵼ��_________________________________��
��4����������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽΪ______________________________��
��10�֣���1��
��2��ʹβ���е�NO��NO2��������ա�Ca(OH)2
��3���ŷ�������NO�������ߡ� ��ƷCa(NO2)2��Ca(NO3)2��������
��4��3NO2����2H��===NO3����2NO����H2O
��2��ʹβ���е�NO��NO2��������ա�Ca(OH)2
��3���ŷ�������NO�������ߡ� ��ƷCa(NO2)2��Ca(NO3)2��������
��4��3NO2����2H��===NO3����2NO����H2O
������Ҫ���鹤ҵ��ȡCa(NO2)2�����̷�����
��1��ƽ�ⳣ������ʽ����������Ũ�ȵ���ָ���Ļ����Է�Ӧ��Ũ�ȵ���ָ���Ļ���
��2����������β����Ŀ���dz�ֽӴ����ա�δ��Ӧ��ʯ����������������ѭ����������β����
��3����NO��NO2Ϊ1��1����Ca(OH)2����ʱ�ķ�Ӧ����ʽΪ��NO��NO2��Ca(OH)2===Ca(NO2)2��H2O, NO2��Ca(OH)2���յķ�Ӧ����ʽΪ4NO2��2Ca(OH)2===Ca(NO2)2��Ca(NO3)2��2H2O��ע��NO���ܵ�����Ca(OH)2���գ����Ե�NO������NO2��ʱ��������NO���ܱ����ն���β���к������ࡣ��NO2��NO������ʱ��������NO2��������Ca(OH)2��Ӧ����Ca(NO3)2��ʹ�ò�Ʒ������
��4��Ca(NO2)2��NΪ��3�ۣ�����NO��NΪ��2�ۣ����ݻ��ϼ�������ֵ��ȣ�����֪�����У�5�۵�N���ɣ�ӦΪNO3�������ݵ�ʧ�����غ����ƽ��NO3����NOǰ�Ļ�ѧ���������ٸ��ݵ���غ㣬ȷ������Ӧǰ��H���μӡ�
��1��ƽ�ⳣ������ʽ����������Ũ�ȵ���ָ���Ļ����Է�Ӧ��Ũ�ȵ���ָ���Ļ���
��2����������β����Ŀ���dz�ֽӴ����ա�δ��Ӧ��ʯ����������������ѭ����������β����
��3����NO��NO2Ϊ1��1����Ca(OH)2����ʱ�ķ�Ӧ����ʽΪ��NO��NO2��Ca(OH)2===Ca(NO2)2��H2O, NO2��Ca(OH)2���յķ�Ӧ����ʽΪ4NO2��2Ca(OH)2===Ca(NO2)2��Ca(NO3)2��2H2O��ע��NO���ܵ�����Ca(OH)2���գ����Ե�NO������NO2��ʱ��������NO���ܱ����ն���β���к������ࡣ��NO2��NO������ʱ��������NO2��������Ca(OH)2��Ӧ����Ca(NO3)2��ʹ�ò�Ʒ������
��4��Ca(NO2)2��NΪ��3�ۣ�����NO��NΪ��2�ۣ����ݻ��ϼ�������ֵ��ȣ�����֪�����У�5�۵�N���ɣ�ӦΪNO3�������ݵ�ʧ�����غ����ƽ��NO3����NOǰ�Ļ�ѧ���������ٸ��ݵ���غ㣬ȷ������Ӧǰ��H���μӡ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
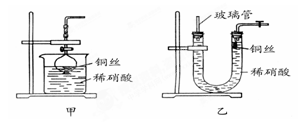
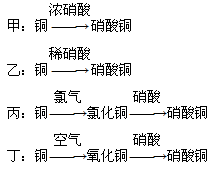
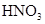 ��
��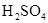 ���ʵ���Ũ�ȷֱ�Ϊ
���ʵ���Ũ�ȷֱ�Ϊ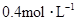 ��
��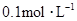 �������Һ�м���1.92gͭ�ۣ����ȣ�����ַ�Ӧ��������Һ��
�������Һ�м���1.92gͭ�ۣ����ȣ�����ַ�Ӧ��������Һ�� ���ʵ���Ũ�ȣ�
���ʵ���Ũ�ȣ�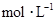 ��Ϊ������
�������� CuO
CuO 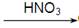 Cu(NO3)2
Cu(NO3)2 CuO
CuO 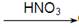 Cu(NO3)2
Cu(NO3)2 =====
=====