��Ŀ����
3�����ݷ�Ӧ����2Fe3++2I-�T2Fe2++I2����Br2+2Fe2+�T2Fe3++2Br-���ش��������⣮��1�����ж����ӵĻ�ԭ����ǿ������˳����B������ĸ��
A��Br-��Fe2+��I-B��I-��Fe2+��Br-C��Br-��I-��Fe2+D��Fe2+��I-��Br-
��2��ij��Һ�к���Br-��Fe2+��I-��Ϊ������I-����Ӱ��Br-��Fe2+��ѡ�õ���������FeBr3Ϊ������Fe2+��I-����Ӱ��Br-��ѡ�õ���������Br2�����ж����뵥���巴Ӧ�IJ���FFeBr3��������������Br2��Fe3+�����뵥�ʵⷴӦ�IJ�����FeI2��������������Fe3+��I2��
��3��I2��Br-�ܷ�����Ӧ����������������Br2��I2��
���� ��1�����Է����е�������ԭ��Ӧ�У���ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�ԣ��ݴ˷������
��2��Ϊ������I-����Ӱ��Br-��Fe2+����������������������Ӧ����I2����С��Br2������Fe2+����Ӧ��Ϊ������Fe2+��I-����Ӱ��Br-������ѡ�Լ��������Դ��������ӡ��ⵥ�ʣ���������Դ��������ӣ��������巴Ӧ�����廯�������������ӵ������Դ��ڵⵥ�ʣ�������ⵥ�ʷ�Ӧ���ɵ⻯������
��3�������嵥�ʵ������Դ��ڵⵥ�ʣ���ⵥ���������Ӳ��ܷ�Ӧ��
��� �⣺��1������2Fe3++2I-=2Fe2++I2�У��÷�Ӧ�л�ԭ����I-����ԭ�������������ӣ���ԭ��I-��Fe2+��
��2Fe2++Br2=2Fe3++2Br-�У���ԭ����Fe2+����ԭ������Br-����ԭ��Fe2+��Br-��
���Ի�ԭ��ǿ��˳���ǣ�I-��Fe2+��Br-����B��ȷ��
�ʴ�Ϊ��B��
��2��������Br2��Fe3+��I2����ԭ�Դ�С˳����I-��Fe2+��Br-��Ϊ������I -����ʹFe2+����������Ӧ��ѡȡ���Σ���ΪFeBr3�����������������ʣ�
Ϊ������Fe2+��I-����Ӱ��Br-��ѡ�õ���������������Ӧ�ô���Fe3+��I2������ѡ��Br2����������
����������Br2��Fe3+���������巴Ӧ����FeBr3������������Fe3+��I2��������ⵥ�ʷ�Ӧ����FeI2��
�ʴ�Ϊ��FeBr3��Br2��FeBr3��������Br2��Fe3+��FeI2��������Fe3+��I2��
��3������������Br2��I2����I2��Br-֮�䲻������Ӧ��
�ʴ�Ϊ����������Br2��I2��
���� ���⿼���������ԡ���ԭ��ǿ���ıȽϣ���Ŀ�Ѷ��еȣ���ȷ������ԭ��Ӧ�������ԡ���ԭ��ǿ�����жϼ�Ӧ�÷���Ϊ���ؼ�������������ѧ���ķ������������Ӧ��������

 ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д�| A�� | 8g | B�� | 18g | C�� | 28g | D�� | 30g |
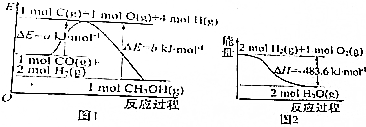
| A�� | 1mol Na0H�ֱ��1mol CH3COOH��1mol HN03��Ӧ�����߱�ǰ�ߡ�HС | |
| B�� | ����ͼ1��֪�ϳɼ״����Ȼ�ѧ����ʽΪCO��g��+2H2��g��=CH30H��g������H1=��b-a��kJ•mol-1 | |
| C�� | ͼ2��ʾ2molH2��g�������е�������2mol��̬ˮ�����е�������483.6kJ | |
| D�� | ����ȼ��ʱ��ȫ���Ļ�ѧ��ת��Ϊ���� |
| A�� | ���ɳ����ķ�Ӧ | B�� | �е��ʲ���ķ�Ӧ | ||
| C�� | �绯��Ӧ | D�� | ���ֽⷴӦ |
| A�� | ������Ӧ�У�SiԪ�ػ��ϼ۲��� | B�� | �����辧���к���Si4+ | ||
| C�� | Si3N4���ڵ����� | D�� | ������Ӧ����������ԭ��Ӧ |