��Ŀ����
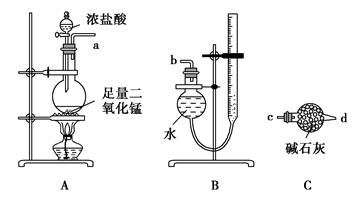
ij��ͬѧ����ͼ��ʾ��װ�òⶨ�����������ĺ�����ҩ���Ϸ������ƣ��ձ���װ��ˮ�����õ��ɼм�ס�ܡ���ȼ�ƣ�����ƿ�в�����ƿ��������Ϩ����ȴ���ɼУ��۲���ƿ��ˮ��仯�����
��1��д��ȼ�ճ��з�����Ӧ�Ļ�ѧ����ʽ�� ����������������ƿ��ˮ�У����ɵ����������뻹ԭ�������ʵ���֮��Ϊ�� ��
��2��ʵ����ϣ����ƿ��ˮ����������С�ڿ��������1/5������һ���������ȷ���ǣ�
a����ͬѧ����δ����ƿ����������Ϩ��ʱ����������ƿ��
b����ͬѧ����ʹ���Ƶ�������
c����ͬѧ����û�н����ɼУ���ȼ��ʱ���ֿ������ȴӵ����ݳ�
d����ͬѧ���ܲ���ȼ�ճ�̫��������ƿ��ǰƿ�ڲ��ֿ��������ݳ�
��1��2Na+O2 Na2O2��1:2 ��2��ab
Na2O2��1:2 ��2��ab
���������������ʵ�����ý�����ȼ�����ļ���ƿ����ƿ��������ѹǿ��С���ظ����º��ɼУ��ձ��ڵ�ˮ���뼯��ƿ��ˮ������������ĵ������������������ȼ�շ�Ӧ����Na2O2��2Na2O2+2H2O=4NaOH+O2��Na2O2�������������ǻ�ԭ��������������������NaOH��һ���ǻ�ԭ������������뻹ԭ�������ʵ���֮��Ϊ1:2�����ˮ�����С��˵�����������ƫС��a��������������װ�ã�������ȥ��ˮƫ�٣���ȷ��b���Ʋ��㣬û�а����������꣬��������٣���ȷ��c�����������ˮ������Ӧ�ø��࣬����d�����������ˮ������Ӧ�ø��࣬����
���㣺�������������������������Ƽ�����������ʡ�������ԭ��Ӧ

����ʵ�鷽�����ܴﵽʵ��Ŀ�ĵ���
| | ʵ��Ŀ�� | ʵ�鷽�� |
| A | �о������Թ�������ֽ����ʵ�Ӱ�� | �ֱ�����֧�Թ��м�����������Ũ�ȵĹ���������Һ����������һֻ�Թ��м�������MnO2 |
| B | ֤��Mg(OH)2��������ת��ΪFe(OH)3���� | ��2mol/LNaOH��Һ���ȼ���3��1mol/L MgCl2��Һ���ټ���3��1mol/L FeCl3��Һ |
| C | ���Լ�����������Һ | ��Na2CO3��Һ��HCl��Һ��μ� |
| D | �ⶨ�������������ĺ��� | ȡa g����������ϡ�����ַ�Ӧ�����ݳ�������ͨ����ʯ�Һ������ΪbL����״���£� |
ʵ������Ҫ������NaCl��Һ����ʵ���ҵ�NaCl�����������Na2SO4��NH4HCO3��ijͬѧ����������ͼ���ʵ���ȥ���ʣ��ش��������⣺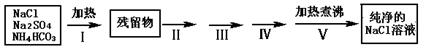
��1������I��ȥ�������ǣ��ѧʽ��_______________��ֱ�Ӽ���Ҫ���ڼ�ǿ����ٽ��м��ȣ������� ��
��2��������ͼ���ʵ����ƣ�����ص�ʵ�������ʵ�������ʵ��Ŀ����д���±��У�
| �������� | ʵ������ | ʵ��Ŀ�� |
| ����II�����������ܽ�õ���Һ�� | | |
| ����III�� |  | |
| ����IV�����ˣ�����Һ�� | | |
| ����V������Һ������� |  | |
��3�������õ�20���NaCl������Һ����֪20��ʱNaCl���ܽ��Ϊ36.0g��NaCl������Һ���ܶ�Ϊ1.12g/cm3 ����20���NaCl������Һ�����ʵ���Ũ��Ϊ mol/L��������������λ��Ч���֣���
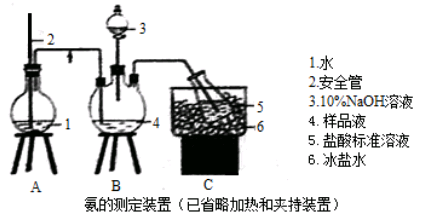
 2CuCl2��O2�ⶨͭ�Ľ������ԭ���������ɹ�ѡ���װ����ͼ��ʾ��
2CuCl2��O2�ⶨͭ�Ľ������ԭ���������ɹ�ѡ���װ����ͼ��ʾ��