��Ŀ����
����Ŀ��������һ���������ɫ��Դ��
��1����101KP�£�1g������ȫȼ������Һ̬ˮ�ų�142.9kJ����������ش��������⣺
��������ȼ����Ϊ________________
�ڸ÷�Ӧ���Ȼ�ѧ����ʽΪ_____________________
��2�����ܵĴ洢���������õ�ǰ�ᣬ��ѧ���о���һ�ִ���Ͻ�Mg2Ni����֪��Mg(s)��H2(g)=MgH2(s)��H1����74.5kJ��mol��1,Mg2Ni(s)��2H2(g)=Mg2NiH4(s)��H2,Mg2Ni(s)��2MgH2(s)=2Mg(s)��Mg2NiH4(s) ��H3��+84.6kJ��mol��1,��H2��____________kJ��mol��1��
���𰸡�-285.8 kJ��mol��12H2(g)+ O2(g)= 2H2O(l)��H����571.6kJ��mol��1��64.4kJ��mol��1
��������
��1����ʵ���ã�1g����ȼ������Һ̬ˮ�ų�142.9kJ������1mol��������2g����ȫ��Ӧ����Һ̬ˮ����Ϊ285.5kJ����������ȼ����285.5kJ/mol����ȷ���� 285.5kJ/mol��
�����ʾ����ȼ�յ��Ȼ�ѧ����ʽΪ��H2(g)+![]() O2(g)=H2O(l)��H=-285.8kJ/mol��2H2(g)+ O2(g)= 2H2O(l) ��H����571.6kJ/mol����ȷ���� H2(g)+
O2(g)=H2O(l)��H=-285.8kJ/mol��2H2(g)+ O2(g)= 2H2O(l) ��H����571.6kJ/mol����ȷ���� H2(g)+![]() O2(g)=H2O(l)��H=-285.8KJ/mol��2H2(g)+ O2(g)= 2H2O(l) ��H����571.6kJ/mol��
O2(g)=H2O(l)��H=-285.8KJ/mol��2H2(g)+ O2(g)= 2H2O(l) ��H����571.6kJ/mol��
��2����Mg(s)��H2(g)=MgH2(s)��H1����74.5kJ��mol��1����Mg2Ni(s)��2MgH2(s)=2Mg(s)��Mg2NiH4(s) ��H3��+84.6kJ��mol��1, �ɸ�˹����2����+���õ�Mg2Ni(s)+2H2(g)�TMg2NiH4(s) ��H2=(-74.5kJ/mol)��2+(84.6kJ/mol)=-64.4kJ/mol������H2=-64.4kJ/mol����ȷ������64.4kJ��mol��1 ��

 ���100�ֵ�Ԫ�Ż�������ϵ�д�
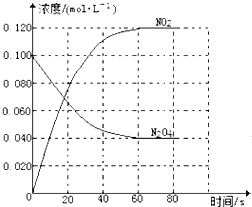
���100�ֵ�Ԫ�Ż�������ϵ�д�����Ŀ���ڲ�ͬ�¶��£���V L�ܱ������м���0.5 mol NO��0.5 mol����̿��������Ӧ��
2NO(g)+C(s)![]() N2(g)+CO2(g) ��H= ��Q kJ��mol-1(Q>0)���ﵽƽ��ʱ���������£�
N2(g)+CO2(g) ��H= ��Q kJ��mol-1(Q>0)���ﵽƽ��ʱ���������£�
�¶ȣ��� | n (C)/mol | n(CO2)/mol |
T1 | 0.15 | |
T2 | 0.375 |
�����й�˵����ȷ����
A. ��������Ϣ����֪��T1 > T2
B. T2��ʱ������Ӧ��ƽ�������С�����������c (N2)��c (NO)����
C. T1��ʱ������ʼʱ��Ӧ�����������Сһ�룬ƽ���NO��ת��������
D. T1��ʱ���÷�Ӧ��ƽ�ⳣ��![]()