��Ŀ����
��CuO�ǽ���ҵ��ͭ����ͭ�Ͻ�ȸ��±��ն��ɵģ�������Ҫ�����������P��ɳ���Դ�CuOΪԭ���Ʊ���������Ҫ�������£�
��֪Fe3+��Fe2+ ��Cu2+ת��Ϊ��Ӧ��������ʱ����ʼ�����ͳ�����ȫʱ��pH���±���
| | Fe3+ | Fe2+ | Cu2+ |
| ��ʼ����ʱ��pH | 2.7 | 7.6 | 5.2 |
| ��ȫ����ʱ��pH | 3.7 | 9.6 | 6.4 |
��1������3% H2O2֮ǰ������в���������������� ��
H2O2�μӷ�Ӧ�����ӷ���ʽΪ ��
��2����ϡ��ˮ����pH��Ŀ���� ��pHӦ���� ��Χ���������ʿ��������ϡ��ˮ���� ��
��NaOH ��Fe2O3 ��CuO ��Na2CO3
��3����������õ��ֵ�����������õ����µ���������������ͬ��������� �� ��
���ˡ� ������Ȳ��衣
��12�֣�ÿ��2�֣���1����ȴ��1�֣��� 2Fe2+ + H2O2 + 2H+�� 2Fe3+ +2H2O��2�֣�
��2��ʹFe3+��ȫת���Fe(OH)3������2�֣���3.7��5.2 ��2�֣� ���ۣ�2�֣�
��4������Ũ����1�֣������½ᾧ��1�֣���ϴ�ӣ�1�֣�
���������������1������˫��ˮ���ȶ��������ֽ�����������ˮ�������ڼ���˫��ˮ֮ǰ��Һ���¶Ƚϸߣ����Լ���3% H2O2֮ǰ���������ȴ�����������������ӳ�����pH�ϴ����Ա��뽫�������������ӳ�������˼���˫��ˮ��Ŀ���������������ӣ���Ӧ�����ӷ���ʽΪ2Fe2+ + H2O2 + 2H+�� 2Fe3+ +2H2O��
��2��Ҫ�õ��������ͱ����ȥ��Һ�е������ӣ�����ݳ���ʱ��pHֵ��֪��������ҺpH��Ŀ����ʹFe3+��ȫת���Fe(OH)3��������������ȫ����ʱ��pH��3.7����ͭ���ӿ�ʼ����ʱ��pH��5.2��������Һ��pHӦ���ڵ�3.7��5.2֮�䡣�����ڵ���pH��ͬʱ�������������ʣ����Ӧ��ѡ������ͭ��������Һ��pH������ѡ�ۡ�
��3������Һ�����������������ͨ������Ũ�������½ᾧ�����ˡ�ϴ�ӡ�����Ȳ������衣
���㣺���鵨���Ʊ�ʵ�������̽�������ʵķ������ᴿ�Լ��ܽ�ƽ���Ӧ�õ�

�������ƣ�NaNO2������Ҫ�ķ�������ij��ѧ��ȤС�鳢���Ʊ��������ƣ��������ϣ���HNO2Ϊ���ᣬ��������Һ�У�NO2-�ɽ�MnO4-��ԭΪMn2�������������ɡ�
��NO����Ӧ���ɱ�����KMnO4��Һ����Ϊ����
̽��һ �������ƹ�����Ʊ�
��̼��Ũ����Ϊ��ʼԭ�ϣ��������װ������һ��������������Ʒ�Ӧ�Ʊ��������ơ�����Ӧ����ʽΪ2NO��Na2O2=2NaNO2�����ּг�װ�ú�A�м���װ�����ԣ�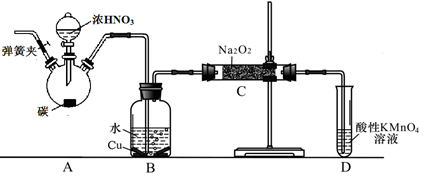
��1��д��װ��A��ƿ�з�����Ӧ�Ļ�ѧ����ʽ ��
��2����ͬѧ��Ϊװ��C�в��ﲻ�����������ƣ�����̼���ƺ��������ƣ�Ϊ�ų�����Ӧ��B��Cװ�ü�����װ��E��E��ʢ�ŵ��Լ�Ӧ�� ������ĸ����
A��ŨH2SO4 B����ʯ�� C����ˮCaCl2
̽���� �������ƹ��庬���IJⶨ��������֤
��ȡװ��C�з�Ӧ��Ĺ���4.000g����ˮ���250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.1000
mol/L����KMnO4��Һ���еζ���ʵ�������������±���ʾ��
| ����� | 1 | 2 | 3 | 4 |
| KMnO4��Һ���/mL | 20.60 | 20.02 | 20.00 | 19.98 |
��3����һ��ʵ�����ݳ����쳣����������쳣��ԭ������� ������ĸ����
A����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
B����ƿϴ����δ����
C���ζ��������Ӷ���
��4�����ݱ������ݣ��������ù������������Ƶ��������� ��
��5����������������ˮ����0.2mol��L-1������������Һ��0.1mol��L-1������������ϣ���Ϻ���Һ�����ԣ����Ϻ���Һ������Ũ���ɴ�С��˳��Ϊ ��
̽���� ��Ӧ��Һ�Ĵ���
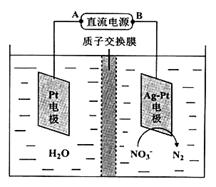
��Ӧ����ƿA����Ȼ����һ���������ᣬ����ֱ���ŷţ���NaOH��Һ�������ԣ����õ绯ѧ���ⷨ���д�����25��ʱ����Ӧ����10min����Һ��pH��7��Ϊ12���绯ѧ����NO3-��ԭ������ͼ��ʾ��
��6����Դ����Ϊ ����A��B����������ӦʽΪ ��
��1��ijѧϰС����0.80mol/L��Ũ�ȵ��ռ���Һ�ⶨδ֪Ũ�ȵ����ᡣ
�ٵζ�����ͼ��ʾ���� �ζ���ʢװ��Ũ�ȵ�����������Һ����ס����ҡ�����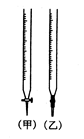
���õζ��ķ������ⶨ�����Ũ�ȣ�ʵ������������ʾ��
| ʵ���� | ����HCl��Һ�����(mL) | ����NaOH��Һ�����(mL) |
| 1 | 20.00 | 23.00 |
| 2 | 20.00 | 23.10 |
| 3 | 20.00 | 22.90 |
��δ֪�����Ũ��Ϊ��������λ��Ч���֣�_______________��
�����в�����ʹ����õ������Ũ��ƫ�͵���__________��
A��ʢװ����Һ����ƿ��ˮϴ��δ����
B���ζ�ǰ����ʽ�ζ��ܼ�������ݣ��ζ���������ʧ
C����ʽ�ζ���������ˮϴ����δ�ñ�����������Һ��ϴ
D������ʽ�ζ��ܵĿ̶�ʱ���ζ�ǰ���Ӱ�Һ����ʹ����ζ����Ӷ���
��2��ij����С��Ϊ�˲ⶨij�Ȼ���(SrCl2)��Ʒ�Ĵ��ȣ���������·�������ȡ1.0 g��Ʒ�ܽ�������ˮ�У������м��뺬AgNO3 2.38 g��AgNO3��Һ(��Һ�г�Cl���⣬����������Ag����Ӧ���ɳ���������)��Cl������ȫ��������Ȼ���ú�Fe3������Һ��ָʾ������0.2 mol��L��1��NH4SCN����Һ�ζ�ʣ���AgNO3��ʹʣ���Ag����AgSCN��ɫ��������ʽ�������Բⶨ�Ȼ�����Ʒ�Ĵ��ȡ�
��ش��������⣺
���жϵζ��ﵽ�յ��������_______________________________________________��
�ڿ���Ag����Fe3������������Һ�еĴ�����ʽ����ʵʩ�ζ�����Һ�Գ�_____(ѡ����ԡ��������ԡ����ԡ�)Ϊ�ˡ�
�����յ㵽��֮ǰ�ĵζ������У����ֳ����������������Ag�����費�Ͼ���ҡ����ƿ�������ʹn(Cl��)�IJⶨ���________(ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
�ζ�ʵ���ǻ�ѧѧ������Ҫ�Ķ���ʵ�顣
��ش��������⣺
��1������к͵ζ������ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ�����в�����ɲⶨ���ƫ�ߵ��� (��ѡ����ĸ)
A���ζ��յ����ʱ�����ӵζ��̶ܿȣ�����������ȷ��
B��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ
D���ζ�ǰ���ζ��ܼ��������ݣ��ζ���������ʧ
��2��������ԭ�ζ�����ȡ������Һ������ƿ�У���������ϡ���ᣬ��Ũ��Ϊ0.1mol��L��1�ĸ��������Һ�ζ��������ķ�ӦΪ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O�������м�¼��ʵ�����ݣ�
| �ζ����� | ����Һ��� (mL) | ��KMnO4��Һ���(mL) | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 25.00 | 0.50 | 20.40 |
| �ڶ��� | 25.00 | 3.00 | 23.00 |
| ������ | 25.00 | 4.00 | 24.10 |
�ٵζ�ʱ��KMnO4��ҺӦװ�� (��ᡱ�)ʽ�ζ����У��ζ��յ�ʱ�ζ�������
�ڸò�����Һ�����ʵ���Ũ��Ϊ_____________��
��3�������ζ��D�D�ζ����ͱ��ζ����������ȵζ�����ָʾ��������������ܡ�
�ο��±��е����ݣ�����AgNO3�ζ�NaSCN��Һ����ѡ�õ�ָʾ���� (��ѡ����ĸ)��
| ������ | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
| ��ɫ | �� | dz�� | �� | ש�� | �� |
| Ksp | 1.77��10��10 | 5.35��10��13 | 1.21��10��16 | 1.12��10��12 | 1.0��10��12 |
A��NaCl B��NaBr C��NaCN D��Na2CrO4
��ͼ��ʾ��װ���У�����a�ܴ������û���H2��bΪ̼�������ڴ�װ�õĸ��������в���ȷ���ǣ� ��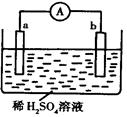
| A��̼����������ų�����ҺpHֵ��� |
| B��a��������b�Ǹ��� |
| C���������е������������Ӵ�a����b�� |
| D��a���Ϸ�����������Ӧ |
����ͼ��ʾ����пƬ��ͭƬ�õ������������װ��ϡ������Һ���ձ��й���ԭ��ء�����������ȷ����
| A��Zn�Ǹ�����������ԭ��Ӧ |
| B��������пƬ����ͭƬ |
| C��һ��ʱ���ͭƬ�������� |
| D����װ�ý���ѧ��ת��Ϊ���� |
һ��ԭ��ص��ܷ�Ӧ�����ӷ���ʽ�ǣ�Zn+Cu2+=Zn2++Cu���÷�Ӧ��ԭ��ص���ȷ�����
| | ���� | ���� | �������Һ |
| A | Zn | Cu | CuCl2 |
| B | Zn | Cu | ZnCl2 |
| C | Cu | Zn | CuSO4 |
| D | Cu | Zn | ZnSO4 |
ijԭ���װ������ͼ��ʾ�������й������У���ȷ����
A�� ������������������Ӧ ������������������Ӧ | B������һ��ʱ������ձ����ܽ� ������ ������ |
C��������Ӧ��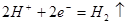 | D������һ��ʱ���NaCl��Һ��c(Cl-)���� |
