��Ŀ����
����˵����ȷ����
| A��NaHCO3��Na2CO3�����Һ�У�һ���� c(Na��)��c(H��)��c(OH��)��c(HCO3-) ��c(CO32-) |
| B��Ũ�Ⱦ�Ϊ0.1 mol��L��1��������Һ��pH�ɴ�С����˳��Ϊ NaOH>Na2CO3>NaHSO4>(NH4)2SO4 |
| C�������������μ�ˮ����Һ�ĵ����ԡ�����ĵ���̶ȡ�pH����������С |
| D��pH=3�������������Һ��c(SO42��)��c(CH3COO��)֮��Ϊ1��2 |
D
�������������A������ȷ������غ�ӦΪ��c(Na��)��c(H��)��c(OH��)��c(HCO3-) ��2c(CO32-)��B������ȷ��NaOH��ǿ����ʣ���ȫ���룬c��OH�� ��=0.1mol��L��1������pH=13��Na2CO3��ǿ�������Σ�ˮ��Һ���ʼ��ԣ�pHС��13��(NH4)2SO4��ǿ�������Σ�ˮ�����Һ�����ԣ�1��pHֵ��7��NaHSO4��ǿ����ʽ�Σ���ˮ����ȫ����������ӡ���������ӡ������ӣ�����C��H����=0.1mol��L��1������pH=1������pH�ɴ�С����˳��ΪNaOH>Na2CO3>(NH4)2SO4>NaHSO4��C������ȷ���ڱ���������μ�ˮ���������ܽⲢ���룬����̶�����������Ũ�����������ڣ���������ϡ�ͣ�����Ũ�ȼ�С������Һ�ĵ����Խ���С������̶�����Һ��ϡ�ͣ�Խ��Խ����Һ��pH��������С��D����ȷ�� pH=3��H2SO4��c(H�� )=0.001mol/L,c(SO42�D )=0.0005mol/L,C(CH3COO�D)=0.001mol/L��c(SO42��)��c(CH3COO��)֮��Ϊ1��2��ѡD��
���㣺�����Һ

W��X��Y��Z��ԭ������������������ֶ�����Ԫ�أ�������WX������ˮ�ĵ��룬���ӻ�����Y2Z����ˮ�ĵ��롣��YԪ����
| A��Li | B��F | C��Na | D��Cl |
�����ʵ���Ũ�ȵ�����������Һ�У� Ũ�������ǣ� ��
Ũ�������ǣ� ��
| A��NH4Cl | B��NH4HCO3 | C��NH4NO3 | D��NH4HSO4 |
��ˮ�ĵ���ƽ��һ��������Ӱ�������
A�� | B�� | C��1s22s22p6 | D��K+ |
����(NH4)2SO4��Һ������˵����ȷ����
A����Һ�д��ڵ���ƽ�⣺(NH4)2SO4 2NH4+ + SO42�� 2NH4+ + SO42�� |
| B������Һ�еμ�����Ũ���ᣬc(NH4+)��c(SO42��)������ |
| C����ˮϡ�ͣ���Һ���������ӵ�Ũ�Ⱦ���С |
| D����Һ�У�c(SO42��)��2c(NH4+) + 2c(NH3��H2O) |
��ʵ�����������ñ�Ũ�ȵ�����ζ�δ֪Ũ�ȵ�̼���ƣ�ͨ��pH����������������ͼ��ͨ����ͼ��ķ���������Ϊ�����ƶ���ȷ����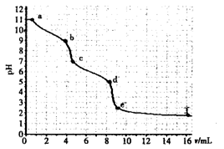
| A��̼���Ƶ�Ũ��Ϊ0.001mol��L��1 |
| B����pH=7ʱ��Һ��[Na��]=[Cl��] |
| C����pH=6ʱ��[Na+]��[HCO3��]��[CO32��] |
| D��c��d��������Ҫ���ӷ�Ӧ��CO32��+H+=HCO3�� |
������Һ�и�����Ũ�ȹ�ϵ��ȷ����
| A�����ʵ���Ũ����ȵĢ�(NH4)2CO3��(NH4)2SO4��(NH4)2Fe(SO4)2������Һ�� c (NH4+)�Ĵ�С˳��Ϊ����>��>�� |
| B��pH��ȵ�NaF��CH3COOK��Һ��c(Na��)��c(F ��)>c(K��)��c(CH3COO��) |
| C��0.2 mo1��L ��1��Na2CO3��Һ��c(OH��)��c(HCO3��)��c(H+)��2c(H2CO3) |
| D��0.2 mo1��L ��1 HCl��0.1 mo1��L ��1 NaAlO2��Һ�������ϣ�c(Cl��)> c(Na+)>c(Al3��)>c(H��)>c(OH��) |
�ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ�����и������У���������ʵ��������
| A��������ˮ�����ζ��ܺ�װ���������еζ� |
| B��������ˮ������ƿ������NaOHҺ��ϴ������װ��һ�������NaOH��Һ |
| C���ü�����ָʾ��������Һ�ɻ�ɫ��ɳ�ɫ�����̶������������ |
| D���ü�ʽ�ζ���ȡ10.00 mLNaOH��Һ����������ˮϴ������ƿ�У��ټ�����������ˮ���еζ� |
���Ȼ�粒����ܽ���D2O����ˮ���У���Ӧ�����ӷ���ʽ��ȷ����
A��NH4+ + D2O  NH3��D2O + H+ NH3��D2O + H+ | B��NH4+ + D2O  NH3��HDO + D+ NH3��HDO + D+ |
C��NH4+ + 2D2O  NH3��HDO + D3O+ NH3��HDO + D3O+ | D��NH4+ + 2D2O  NH3��D2O + HD2O+ NH3��D2O + HD2O+ |