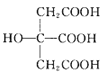
��Ŀ����
����Ŀ��ij��ѧС���ͬѧȡһ������Al��Fe2O3�Ļ����������ȷ�Ӧ����̽��������ijɷ֡���ش��������⣺
��.��1���������ȷ�Ӧ��ʵ�������_____________________
��2�������ȷ�Ӧʱ���ڲ�ֽ©���ײ���һС����ˮ��ʪ��Ŀ����_________________
��3����Ӧ�Ļ�ѧ����ʽΪ_____________
��.��֪��Al��Fe���ۡ��е��������£�
���� | Al | Fe |
�۵�(��) | 660 | 1 535 |
�е�(��) | 2 467 | 2 750 |
��1��ijͬѧ�²⣬���ȷ�Ӧ���õ����������������Ͻ����ɣ��÷�Ӧ������ʹ���ۻ����������۵�����ͣ��������������γɺϽ�����Ϊ���Ľ����Ƿ������____________(����������������������)�����һ����ʵ�鷽����֤���������õ��������к��н�������_____________________
���𰸡�������KClO3������Mg���������ȼ�� ʹ���������������ҷ�ֹֽ©���Ż� Fe2O3+2Al![]() 2Fe+Al2O3�� ���� ȡ������ȴ��Ŀ�״�����������Թ��У���������������Һ�����������������������
2Fe+Al2O3�� ���� ȡ������ȴ��Ŀ�״�����������Թ��У���������������Һ�����������������������
��������
��.��1���������ȷ�Ӧ�IJ�������Ϊ��������KClO3������Mg���������ȼ��
��2��Ϊ��ֹֽ©���Ż𣬿ɽ�ֽ©����ˮʪ��
��3����Ӧ�Ļ�ѧ����ʽΪFe2O3�ڸ����·������ȷ�Ӧ����������������
��. ��1���ɽ������ܺ��������Ʒ�Ӧ�ų����������������������Ʋ���Ӧ�ɷ�����
��.��1��Ϊʹ��Ӧ˳�����У��ɼ�������أ�Ϊ��ȼ������ȼþʱ��Ӧ�ɷ�����þΪ��ȼ���������������ȷ�Ӧ��ʵ������Ǽ�����KClO3������þ���������ȼ��
��2�����ȷ�Ӧ�¶Ⱥܸߣ�Ϊ��ֹֽ©���Ż𣬿ɽ�ֽ©����ˮʪ��
��3��Fe2O3�ڸ����·������ȷ�Ӧ������������������Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+2Al![]() 2Fe+Al2O3��
2Fe+Al2O3��
��.��1���÷�Ӧ������ʹ���ۻ����������۵�����ͣ���ʱҺ̬���������ۺ��γ������Ͻ𡣽������ܺ��������Ʒ�Ӧ�ų����������������������Ʋ���Ӧ����Ӧ�����ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2��������������������Һ֤���������õĿ�״�������к��н���������������Ϊ��ȡ������ȴ��Ŀ�״�����������Թ��У���������������Һ������Ϊ�������������