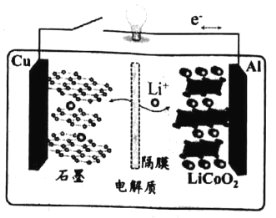
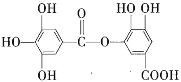
��Ŀ����
����Ŀ����λ����һ������Ļ�ѧ�������й��õ��Ӷ���ijԭ�ӵ������ṩ����![]() ������
������![]() (��ԭ���ṩ���õ��Ӷ�)��
(��ԭ���ṩ���õ��Ӷ�)��![]() (ȱ����)ͨ����λ���γɵġ��ݴ˻ش��������⡣
(ȱ����)ͨ����λ���γɵġ��ݴ˻ش��������⡣
(1)���������д�����λ������__________(�����)
A.![]() B.
B.![]() C.
C.![]() D.
D.![]()
(2)����(![]() )��Һ�����ԣ���д������뷽��ʽ��____________________��
)��Һ�����ԣ���д������뷽��ʽ��____________________��
(3)��ѧ�Ҷ�![]() �ṹ����ʶ�����˽�Ϊ�����Ĺ��̣������ѧ����������ֹ۵㣺
�ṹ����ʶ�����˽�Ϊ�����Ĺ��̣������ѧ����������ֹ۵㣺
�ף�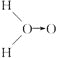 (ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ����)
(ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ����)
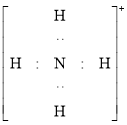
�ң�HOOH
��ѧ��Baeyer��VilliyerΪ�о�H2O2�Ľṹ����Ʋ����������ʵ�飺
a����C2H5OH��ŨH2SO4��Ӧ����(C2H5)2SO4��ˮ��
b�����Ƶõ�(C2H5)2SO4��H2O2��Ӧ��ֻ����A��H2SO4��
c�������ɵ�A��H2��Ӧ(��֪�÷�Ӧ��H2����ԭ��)��
�����H2O2�Ľṹ�����ʾ��ʵ��c�л�ѧ��Ӧ����ʽΪ(Aд�ṹ��ʽ)________________��
��Ϊ�˽�һ��ȷ��H2O2�Ľṹ������Ҫ��ʵ��c������һ��ʵ��d�������d��ʵ�鷽����____________��
���𰸡�BD ![]()
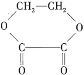
![]() +H2��C2H5OC2H5+H2O ����ˮ����ͭ����c�ķ�Ӧ��������û��ˮ�������������𰸣�
+H2��C2H5OC2H5+H2O ����ˮ����ͭ����c�ķ�Ӧ��������û��ˮ�������������𰸣�
��������
(1)�����������Ƿ����ṩ�¶Ե��ӵ�����������ɵ��ӵĿչ�������������жϣ�
(2)����Ϊȱ���ӷ��ӣ��ܽ��ܹ¶Ե��ӣ�
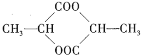
(3)�������⣬��ʵ����Ƶ�˼·Ϊ����C2H5OH��ŨH2SO4��Ӧ���ɵ�(C2H5)2SO4��H2O2��Ӧ����A(C2H5)2O2�����û�ԭ��H2��ԭ������Ƿ�����ˮ���������ˮ��H2O2Ϊ�ṹ������Ϊ�ҽṹ��
(1)������λ���ĸ����֪��Ҫ�γ���λ���������ṩ�¶Ե��ӵ�����������ɹ¶Ե��ӵĿչ����
A. CO2�е�̼����ΪC��O�ṩ���������γɣ�û����λ����
B. H3O+�ɿ�����H2O�е�O�ṩ�¶Ե�����H+�����γɣ���������λ����
C. CH4��C-H��ΪC��H�ṩ���������γɣ�û����λ����
D. H2SO4��S�ͷ��ǻ�O֮������λ����
����BD��
(2)������Bԭ�Ӻ��пչ����ˮ�е���ԭ���ṩ�¶Ե��ӣ��γ���λ����������������ˮ�����ԣ����뷽��ʽΪH3BO3��H2O![]() H����[B(OH)4]����
H����[B(OH)4]����
����H3BO3��H2O![]() H����[B(OH)4]����
H����[B(OH)4]����
(3)����ԭ���غ��֪��A�ķ���ʽΪC4H10O2���������˫��ˮ�Ľṹ�����ʾ��O��O��������ԭ��ʱ���ѣ���c�еķ�ӦΪ![]() +H2��C2H5OC2H5+H2O�����˫��ˮ�Ľṹ������ʾ����ӦΪC2H5O-OC2H5 + H2 �� 2CH3OH�����ߵ�����֮һΪ�Ƿ���ˮ���ɣ����Կ�������ˮ����ͭ���飬�Ӷ������жϡ�
+H2��C2H5OC2H5+H2O�����˫��ˮ�Ľṹ������ʾ����ӦΪC2H5O-OC2H5 + H2 �� 2CH3OH�����ߵ�����֮һΪ�Ƿ���ˮ���ɣ����Կ�������ˮ����ͭ���飬�Ӷ������жϡ�
��Ϊ��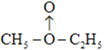 +H2��C2H5OC2H5+H2O������ˮ����ͭ����c�ķ�Ӧ��������û��ˮ(������������)��
+H2��C2H5OC2H5+H2O������ˮ����ͭ����c�ķ�Ӧ��������û��ˮ(������������)��

����Ŀ��S2Cl2��SCl2��Ϊ��Ҫ�Ļ���ԭ�ϣ�������8�����ȶ��ṹ��
��֪:��S2(1)+Cl2(g)![]() S2Cl2(g) ��H1=xkJ/mol
S2Cl2(g) ��H1=xkJ/mol
��S2Cl2(g)+Cl2(g)![]() 2SCl2(g) ��H2=ykJ/mol
2SCl2(g) ��H2=ykJ/mol
����ػ�ѧ���ļ������±���ʾ:
��ѧ�� | S-S | S-Cl | Cl-Cl |
����/kJ/mol | a | b | c |
����˵���������
A. SCl2�ĽṹʽΪC1-S-Cl B. S2Cl2�ĵ���ʽΪ: ![]()
C. y=2b-a-c D. ��S2(1)+2Cl2(g)![]() 2SCl2(g)�ķ�Ӧ�У���H=(x+y)kJ/mol
2SCl2(g)�ķ�Ӧ�У���H=(x+y)kJ/mol