��Ŀ����
20��ij��ȤС���Ա�����ϩΪ��Ҫԭ�ϣ���������·�ߺϳ�ҩ����³����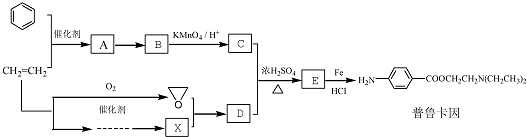
��֪��
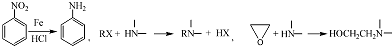
��ش�
��1��A-��B�Ļ�ѧ����ʽ��
 ��
����2��������D�Ľṹ��ʽ��HOCH2CH2N��CH2CH3��2��
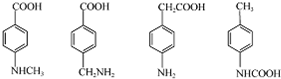
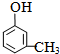
��3��ͬʱ��������������B������ͬ���칹��Ľṹ��ʽ��
 ��
���ٺ��������ʾ�����к����Ȼ���
��1H-NMR����ʾ�����к��б������ұ����������ֲ�ͬ��ѧ��������ԭ�ӣ�
��4���û�ѧ����ʽ��ʾ����ϩΪԭ���Ʊ�X�ĺϳ�·�ߣ����Լ���ѡ��CH2=CH2+HCl$\stackrel{һ��������}{��}$CH3CH2Cl��2CH3CH2Cl+NH3$\stackrel{һ��������}{��}$��CH2CH3��2NH+2HCl��
���� �������Ϣ��֪��E������ԭ��Ӧ����³��������EӦΪ ��C��D��Ũ����������·���������Ӧ��E�����Խ��D����ϩת���ɵĻ��������X������Ϣ�еķ�Ӧ�����ɵIJ������D��Ӧ�����б���������C��Ӧ���б����������ṹ�����Է�Ӧ����ӦΪ��ϩ�뱽�����ӳɷ�Ӧ��A��A����������Ӧ��B��B����������Ӧ��C������CΪ
��C��D��Ũ����������·���������Ӧ��E�����Խ��D����ϩת���ɵĻ��������X������Ϣ�еķ�Ӧ�����ɵIJ������D��Ӧ�����б���������C��Ӧ���б����������ṹ�����Է�Ӧ����ӦΪ��ϩ�뱽�����ӳɷ�Ӧ��A��A����������Ӧ��B��B����������Ӧ��C������CΪ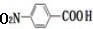 ��BΪ
��BΪ ��AΪ
��AΪ ���Ƚ���³�����C�Ľṹ��֪��DΪHOCH2CH2N��CH2CH3��2���������Ϣ��֪XӦΪ��CH2CH3��2NH����϶�Ӧ�����Լ���ĿҪ������⣮
���Ƚ���³�����C�Ľṹ��֪��DΪHOCH2CH2N��CH2CH3��2���������Ϣ��֪XӦΪ��CH2CH3��2NH����϶�Ӧ�����Լ���ĿҪ������⣮
��� �⣺�������Ϣ��֪��E������ԭ��Ӧ����³��������EӦΪ ��C��D��Ũ����������·���������Ӧ��E�����Խ��D����ϩת���ɵĻ��������X������Ϣ�еķ�Ӧ�����ɵIJ������D��Ӧ�����б���������C��Ӧ���б����������ṹ�����Է�Ӧ����ӦΪ��ϩ�뱽�����ӳɷ�Ӧ��A��A����������Ӧ��B��B����������Ӧ��C������CΪ
��C��D��Ũ����������·���������Ӧ��E�����Խ��D����ϩת���ɵĻ��������X������Ϣ�еķ�Ӧ�����ɵIJ������D��Ӧ�����б���������C��Ӧ���б����������ṹ�����Է�Ӧ����ӦΪ��ϩ�뱽�����ӳɷ�Ӧ��A��A����������Ӧ��B��B����������Ӧ��C������CΪ ��BΪ
��BΪ ��AΪ
��AΪ ���Ƚ���³�����C�Ľṹ��֪��DΪHOCH2CH2N��CH2CH3��2���������Ϣ��֪XӦΪ��CH2CH3��2NH��
���Ƚ���³�����C�Ľṹ��֪��DΪHOCH2CH2N��CH2CH3��2���������Ϣ��֪XӦΪ��CH2CH3��2NH��
��1����������ķ�����֪��A��B�Ļ�ѧ����ʽ�� ��
��
�ʴ�Ϊ�� ��
��
��2����������ķ�����֪��DΪHOCH2CH2N��CH2CH3��2��
�ʴ�Ϊ��HOCH2CH2N��CH2CH3��2��
��3�������������ٺ��������ʾ�����к����Ȼ�����1H-NMR����ʾ�����к��б������ұ����������ֲ�ͬ��ѧ��������ԭ�ӣ������������������ڶ�λ���ţ������������B������ͬ���칹��Ľṹ��ʽ�� ��
��
�ʴ�Ϊ�� ��
��
��4����5�������Ϸ�����֪XӦΪ��CH2CH3��2NH������CH3CH2Cl��NH3��Ӧ���ɣ�����ϩΪԭ�ϣ���Ӧ�Ⱥ�HCl�ӳɣ�����CH3CH2Cl����ط�Ӧ�ķ���ʽΪCH2=CH2+HCl$\stackrel{һ��������}{��}$CH3CH2Cl��2CH3CH2Cl+NH3$\stackrel{һ��������}{��}$��CH2CH3��2NH+2HCl��
�ʴ�Ϊ��CH2=CH2+HCl$\stackrel{һ��������}{��}$CH3CH2Cl��2CH3CH2Cl+NH3$\stackrel{һ��������}{��}$��CH2CH3��2NH+2HCl��
���� ����������л���ĺϳ����ƶϵĿ��飬�ۺϿ���ѧ���ķ��������Ŀ��飬����ʱע����������Ϣ�����չ����ŵ����ʣ��Ѷ��еȣ�

��HF��HCl��HBr��HI�����ȶ������μ��� ��NH3��Һ��
��F2��Cl2��Br2��I2���ۡ��е������� ��H2S���ۡ��е�С��H2O���ۡ��е㣮
| A�� | �٢ۢ� | B�� | �ۢ� | C�� | �ڢۢ� | D�� | ȫ�� |
| A�� | ��ΪH2O�ķе��H2S�ߣ�����Oԭ�ӵõ�����������Sԭ�� | |
| B�� | ��ΪFԭ�ӵõ���������Clԭ��ǿ���������������ǿ�� | |
| C�� | ��ΪH2CO3���Դ���H2SiO3������CH4�ȶ��Դ���SiH4 | |
| D�� | ��ΪNa+KCl�����ڣ�=NaCl+K��������Naԭ��ʧ������������Kԭ�� |
| A�� | HC1�ĵ���ʽ��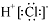 | B�� | CH4�����ģ�ͣ�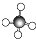 | ||
| C�� | S2-�Ľṹʾ��ͼ�� | D�� | ��ϩ�Ľṹ��ʽ��CH2�TCH2 |
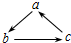 ������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������
������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������| ���� ѡ �� | a | b | c |
| A | Si | SiO2 | H2SiO3 |
| B | H2SO4 | SO2 | SO3 |
| C | Al | AlCl3 | Al��OH��3 |
| D | NaCl | Na | NaOH |
| A�� | A | B�� | B | C�� | C | D�� | D |
| ������/ѡ�� | A | B | C | D |
| Y | H2O | Fe2O3 | C2H5OH | FeCl3 |
| W | Fe3O4 | Al2O3 | C2H5ONa | CuCl2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ��
�� ��
��