��Ŀ����
����Ŀ��������(SO2Cl2)�۵㣭54.1���е�69.2������Ⱦ�ϡ�ҩƷ�����ݼ���ũ��ɱ�������������������Ҫ���á�
��1��SO2Cl2��S�Ļ��ϼ�Ϊ___________��SO2Cl2�ڳ�ʪ��������ˮ�����������Ļ�ѧ����ʽΪ___________��
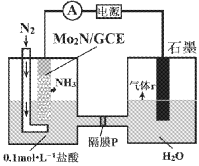
��2�������ø����Cl2��SO2�ڻ���̿������ȡ�����ȣ�ʵ��װ����ͼ��ʾ(���ּг�װ��δ����)��

������A������Ϊ___________��װ������װ����Լ�___________��װ��B��������___________��
��װ�ñ���Һ©����ʢװ������Լ���___________(ѡ����ĸ)��
A.����ˮ B.10.0mol��L��1Ũ���� CŨ����������Һ D����ʳ��ˮ
�۵ζ����ⶨ�����ȵĴ��ȣ�ȡ1.800g��Ʒ�����뵽100mL0.5000mol�� L��1 NaoH��Һ�м��ȳ��ˮ�⣬��ȴ�������ˮȷϡ����250mL��ȡ25mL��Һ����ƿ�У��μ�2�μ��ȣ���0.1000mol��L��1��HCl�ζ����յ㣬�ظ�ʵ������ȡƽ��ֵ������10.00mL���ﵽ�ζ��յ������Ϊ___________����Ʒ�Ĵ���Ϊ___________��
��3��̽���������ڴ��������¼��ȷֽ�IJ��ʵ��װ����ͼ��ʾ(���ּг�װ��δ����)
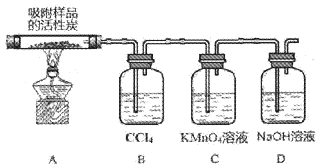
�ټ���ʱA���Թܳ��ֻ���ɫ��װ��B��������___________
��װ��C�е�������___________����Ӧ�����ӷ���ʽΪ___________��
���𰸡�+6 SO2Cl2+2H2O��2HCl��+H2SO4 ���������������� Ũ���� ��ֹ������ˮ��������������ƿ��ʹSO2Cl2����ˮ����ʲ�������β��SO2��Cl2����ֹ��Ⱦ���� D �μ����һ��HCl��Һ����ƿ����Һ�ɻ�ɫ��Ϊ��ɫ�� �Ұ���Ӳ��ָ� 75% ����Cl2 C��KMnO4��Һ��ɫ 2MnO4��+5SO2+2H2O��2Mn2++5SO42��+4H+
��������
��1�����ݻ��ϼ۴�����Ϊ���ԭ��ȷ����Ļ��ϼۣ�SO2Cl2�ڳ�ʪ��������ˮ�⡰���̡�������Ϊ��ˮ��Ӧ�������ӷ���HCl��
��2����װ�ü�������AΪ�����Σ������ܣ�Ϊ����Cl2��װ������װ����Լ���Ũ���ᣬװ��B�������ǣ���ֹ������ˮ��������������ƿ��ʹSO2Cl2����ˮ����ʲ�������β��SO2��Cl2����ֹ��Ⱦ������
����ΪCl2�ڱ���ʳ��ˮ���ܽ��С����������μӱ���ʳ��ˮ��ʹCl2�ӱ����ų���
�����������ȷ���ˮ������ɵ������������������Ʒ�Ӧ�Ĺ�ϵʱ���㣻
��3���������ڴ��������¼��ȷֽ����ɶ��������������SO2ʹKMnO4��Һ��ɫ��
��1�����ݻ��ϼ۴�����Ϊ���ԭ��O��-2�ۣ�Cl��-1��������SO2Cl2��S�Ļ��ϼ�Ϊ![]() 6�ۣ�SO2Cl2�ڳ�ʪ��������ˮ�⡰���̡�������Ϊ��ˮ��Ӧ�������ӷ���HCl����Ӧ�Ļ�ѧ����ʽΪ��SO2Cl2+2H2O��2HCl��+H2SO4��
6�ۣ�SO2Cl2�ڳ�ʪ��������ˮ�⡰���̡�������Ϊ��ˮ��Ӧ�������ӷ���HCl����Ӧ�Ļ�ѧ����ʽΪ��SO2Cl2+2H2O��2HCl��+H2SO4��
�������+6 �� SO2Cl2+2H2O��2HCl��+H2SO4 ��
��2����װ�ü�������AΪ�����Σ������ܡ�Ϊ����Cl2��װ������װ����Լ���Ũ���ᣬװ��B�������ǣ���ֹ������ˮ��������������ƿ��ʹSO2Cl2����ˮ����ʲ�������β��SO2��Cl2����ֹ��Ⱦ������
�����Ϊ�������Σ������ܣ�Ũ���� �� ��ֹ������ˮ��������������ƿ��ʹSO2Cl2����ˮ����ʲ�������β��SO2��Cl2����ֹ��Ⱦ������
����ΪCl2�ڱ���ʳ��ˮ���ܽ��С����������μӱ���ʳ��ˮ��ʹCl2�ӱ����ų���
�������D��
����Ϊ�����ȷ���ˮ������ɵ���������ᣬ�����������ܺ��������Ʒ�Ӧ�������У�SO2Cl2![]() 4NaOH,����Ϊ��Ӧ���ü�����ָʾ����������ζ�������NaOH���ζ��յ�������ǣ��μ����һ��HCl��Һ����ƿ����Һ�ɻ�ɫ��Ϊ��ɫ�� �Ұ���Ӳ��ָ�����������25ml��Һ��n(NaOH)= 0.1000mol��L��1
4NaOH,����Ϊ��Ӧ���ü�����ָʾ����������ζ�������NaOH���ζ��յ�������ǣ��μ����һ��HCl��Һ����ƿ����Һ�ɻ�ɫ��Ϊ��ɫ�� �Ұ���Ӳ��ָ�����������25ml��Һ��n(NaOH)= 0.1000mol��L��1![]() 0.01L=0.001mol,���ԣ�ȡ1.800g��Ʒ�����뵽100mL0.5000mol�� L��1 NaoH��Һ�м��ȳ��ˮ�⣬NaOH����0.01mol����1.800g��Ʒ�к�SO2Cl2�����ʵ���Ϊxmol,��1:4=x����0.1
0.01L=0.001mol,���ԣ�ȡ1.800g��Ʒ�����뵽100mL0.5000mol�� L��1 NaoH��Һ�м��ȳ��ˮ�⣬NaOH����0.01mol����1.800g��Ʒ�к�SO2Cl2�����ʵ���Ϊxmol,��1:4=x����0.1![]() 0.5
0.5![]() 0.01�������x=0.01mol������1.800g��Ʒ�к�SO2Cl2������Ϊ��0.01mol
0.01�������x=0.01mol������1.800g��Ʒ�к�SO2Cl2������Ϊ��0.01mol![]() 135g/mol=1.35g���ʲ�Ʒ�Ĵ���Ϊ:
135g/mol=1.35g���ʲ�Ʒ�Ĵ���Ϊ:![]() =75
=75![]() ��
��
�����Ϊ���μ����һ��HCl��Һ����ƿ����Һ�ɻ�ɫ��Ϊ��ɫ�� �Ұ���Ӳ��ָ���75![]() ��
��
��3���������⣬�������ڴ��������¼��ȷֽ����ɶ���������������ټ���ʱ�����Ļ���ɫ������Cl2������Bװ�õ���������CCl4����Cl2��
�����������Cl2��
��C�е�SO2��KMnO4��Һ����������ԭ��Ӧ��ʹC��KMnO4��Һ��ɫ�����ӷ���ʽΪ��2MnO4��+5SO2+2H2O��2Mn2++5SO42��+4H+��
�����Ϊ��C��KMnO4��Һ��ɫ��2MnO4��+5SO2+2H2O��2Mn2++5SO42��+4H+��

 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�