��Ŀ����
���в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| A | ���������Ӳ㣬K��M�������֮�͵���L������� |
| B | �������н�������ǿ |
| C | �����µ���Ϊ˫ԭ�ӷ��ӣ��⻯���ˮ��Һ�ʼ��� |
| D | Ԫ�����������+7�� |
��AԪ�������ڱ��е�λ�� ��
��BԪ��ԭ�ӽṹʾ��ͼ ��
��C���ʷ��ӵĵ���ʽ ���õ���ʽ��ʾA��BԪ����ɵĻ�������γɹ��� ��
��DԪ������Ȼ�������ֺ��أ���ԭ�ӷ��ű�ʾ��������Ϊ20�ĺ��� ��
��2��Ԫ��D��Ԫ��A��ȣ��ǽ����Խ�ǿ���� ����Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ���� ����ѡ����ţ���
a��������D�ĵ��ʺ�A�ĵ���״̬��ͬ
b��D���⻯���A���⻯���ȶ�
c��һ��������D��A�ĵ��ʶ������Ʒ�Ӧ
d��A����ۺ�������������D����ۺ�����
e��D��������A���⻯�ﷴӦ����A����
��3��̽Ѱ���ʵ����ʲ�������ѧϰ����Ҫ����֮һ��A��B��C��D����Ԫ�ص�����������ˮ�����л�ѧ�������Բ�ͬ���������ֵ��� ��д��ѧʽ����
��4��X����A��B��C��D����Ԫ���е�ij��Ԫ����ɵĵ��ʣ��ܾ���ͼ��ʾ�Ĺ���ת��ΪW������������ȥ����
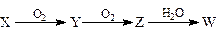
����Y���д̼�����ζ����ɫ���壬��Yͨ��BaCl2��Һ�У�Ȼ��μ�����H2O2��Һ���а�ɫ�������ɣ��˰�ɫ�����Ļ�ѧʽΪ�� �����ɸð�ɫ�����Ļ�ѧ����ʽΪ ��
����Z�Ǻ���ɫ���壬��Z��W�ķ�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ�� ��
��1���������ڢ�A�� ..........................................................��1�֣�
�� ...........................................................��1�֣�
...........................................................��1�֣�
��  ............................................................��1�֣�
............................................................��1�֣� ��.��2�֣�
��.��2�֣�
�� ............................................................................��1�֣�
............................................................................��1�֣�
��2��Cl ...........................................................................��1�֣�
bde ...........................................................................��2�֣�
��3��NaOH ..........................................................................��1�֣�
��4����BaSO4..........................................................................��1�֣�
BaCl2+SO2+H2O2=BaSO4��+2HCl����Ba2+ +SO2 +H2O2 = BaSO4��+2H+��....��2�֣�
��1:2 ..........................................................................��2�֣�
����

 ��У����ϵ�д�
��У����ϵ�д�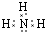
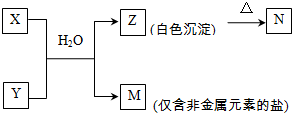
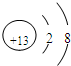
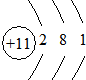
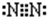
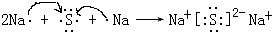
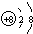
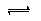 Al��OH��3
Al��OH��3 Al3++3OH-
Al3++3OH-