��Ŀ����
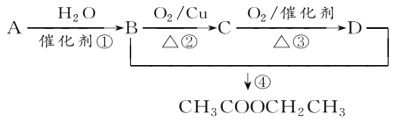
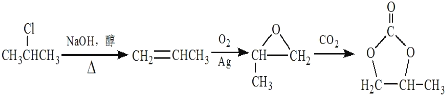
����Ŀ��A(C2H4)�ǻ������л�����ԭ�ϡ���A�ͳ������л���ɺϳ�һ���������Ϻ�һ����ȩ�����ϡ�����ϳ�·����ͼ��ʾ ( ���ַ�Ӧ������ȥ ):
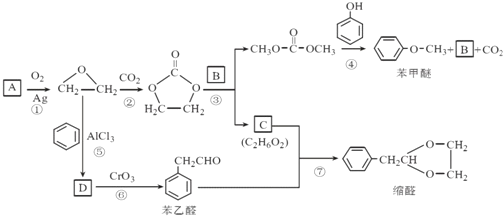
��֪����![]()
��DΪ��ȡ�������廯��������������Ʒ�Ӧ��ÿ��D������ֻ����1����ԭ�ӣ�D����Ԫ�ص���������ԼΪ13.1%.
�ش��������⣺
��1��A�������� ___ ��ͼ����ȩ�ķ���ʽ�� ___.
��2��B�Ľṹ��ʽΪ ___.
��3���ݵĻ�ѧ����ʽΪ ___.
��4���ķ�Ӧ������ ___.
��5����д���˴Ź��������� 4 ��� , �����֮��Ϊ 3:2:2:1, �����б�����![]() �ṹ�ı���ȩ������ͬ���칹��Ľṹ��ʽ�� ___.
�ṹ�ı���ȩ������ͬ���칹��Ľṹ��ʽ�� ___.
��6������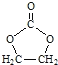 �ĺϳ�·��,���һ����2�ȱ���ͱ�Ҫ�����Լ��Ʊ�
�ĺϳ�·��,���һ����2�ȱ���ͱ�Ҫ�����Լ��Ʊ�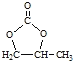 �ĺϳ�·��(ע����Ҫ�ķ�Ӧ���� )___��
�ĺϳ�·��(ע����Ҫ�ķ�Ӧ���� )___��
���𰸡���ϩ C10H12O2 CH3OH 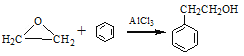 ������Ӧ
������Ӧ ![]() ��
��![]()
��������
��1��A�ķ���ʽΪC2H4����A�ǻ������л�����ԭ�ϣ���AΪ��ϩ��������ȩ�Ľṹ��ʽ���Ƴ���ȩ�ķ���ʽΪC10H12O2��
��2��������ȩ�ṹ��ʽ���Ƴ�C�Ľṹ��ʽΪHOCH2CH2OH��������Ϣ�٣��Ƴ�BΪCH3OH��
��3��D��������Ʒ�Ӧ����D������ֻ��һ����ԭ�ӣ���D�к��У�OH��D���ڷ����廯�����D�к��б�����D����Է�������Ϊ1��16/13.1%=122����DΪ���״������������뱽��AlCl3�����·�����Ӧ���ɱ��״�������ѧ��Ӧ����ʽΪ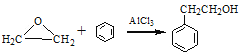 ��
��
��4�������������������״��������ɱ���ȩ������Ӧ��Ϊ������Ӧ��
��5����4��壬˵���ǶԳƽṹ����������������Ϣ�����������Ľṹ��ʽΪ ![]() ��
��![]() ��
��
��6�����ݷ�Ӧ�ڣ��Ƴ�����Ŀ����ԭ����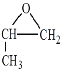 ������·�ߢ٣�����ϩ��O2��Ag�����������������ɻ������飬�������
������·�ߢ٣�����ϩ��O2��Ag�����������������ɻ������飬������� ��ԭ����CH3CH=CH2�����ɱ�ϩ����2�����鷢����ȥ��Ӧ���ϳ�·��Ϊ
��ԭ����CH3CH=CH2�����ɱ�ϩ����2�����鷢����ȥ��Ӧ���ϳ�·��Ϊ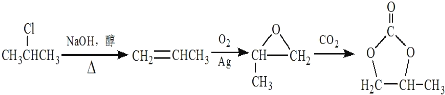 ��
��