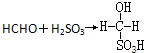��Ŀ����
18��ʵ������Ҫ450ml 0.12mol/L��NaOH��Һ�������²������ٰѳƺõ�NaOH����С�ձ��У�����������ˮ�ܽ⣮
�ڸ��ݼ�����������ƽ��ȡNaOH2.4�ˣ�
�۰Ѣ�������ҺС��ת��һ���ݻ�������ƿ�У�
�ܼ���������ƿ�м�����ˮ��Һ���̶���1cm-2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ����̶������У�
������������ˮϴ���ձ��Ͳ�����2-3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ת������ƿ��������ҡ�ȣ�
������ƿƿ�����������ҡ�ȣ�
����д���пհף�
��1������������Ҫ������NaOH����Ϊ2.4��
��2�������������ȷ˳��Ϊ������ţ��ڢ٢ۢݢܢޣ�
��3��ʵ���������¹�������ƿ����100mL ��250mL ��500mL ��1000mL����ʵ��ѡ�âۣ�����ţ���
��4����ʵ���õ��Ļ���ʵ��������������ƽ��ҩ������У��ձ�����������500mL����ƿ����ͷ�ιܣ�
��5�������������ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�ٳ���NaOH����ʱ�����뵹�ã�1�����������룩ƫ��
��û�н��в��������ƫ��
������ƿ��ԭ��������ˮ��Ӱ��
��ijͬѧ����ʱ��������ƿ�̶���ƫ��
�ݶ��ݣ�ҡ�Ⱥ���Һ����ڿ̶��ߣ��ý�ͷ�ι��ּ�������ˮʹҺ�尼Һ���ٴ���̶�������ƫ�ͣ�
���� ��1����������ƿ��450mL����ƿ����Ӧѡ��500mL����ƿ�����Ƴ�500mL��Һ������m=CVM�����㣻
��2���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���Բ���˳���������
��3����������ƿֻ��һ���̶�����ѡ����ʵ�����ƿ��
��4���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��5������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��� �⣺��1����������ƿ��450mL����ƿ����Ӧѡ��500mL����ƿ�����Ƴ�500mL��Һ0.12mol/LNaOH��Һ������m=CVM��֪���������m=0.12mol/L��0.5L��40g/mol=2.4g���ʴ�Ϊ��2.4��
��2���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪��ȷ��˳��Ϊ���ڢ٢ۢݢܢޣ��ʴ�Ϊ���ڢ٢ۢݢܢޣ�
��3������ƿֻ��һ���̶��ߣ���һ����������ƿֻ�����Ƴ�������Ӧ�������Һ������ʵ������450mL����ƿ��Ӧѡ��500mL������ƿ����ѡ�ۣ�
��4���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ֪������������������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ��ʳ���ҩ��������ƽ�⣬����Ҫ�ձ�����������500mL����ƿ����ͷ�ιܣ��ʴ�Ϊ���ձ�����������500mL����ƿ����ͷ�ιܣ�
��5���ٳ���NaOHʱ��������������̣��ᵼ��ҩƷ������ƫС����������Һ��Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��û�н��в�������ݣ��ᵼ�����ʵ���ʧ������ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죬�ʴ�Ϊ����Ӱ�죻
��ijͬѧ����ʱ��������ƿ�̶��ߣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
�ݶ��ݣ�ҡ�Ⱥ���Һ����ڿ̶����������ģ��ټ�ˮ�ᵼ����ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

 a��b��c��d��e�������ʾ�����һ����ͬ��Ԫ�أ���һ�������¿ɷ�����ͼ��ʾת��������a�ǵ��ʣ�b��ͨ��״���������壬c��d���������e�Ǹ�Ԫ�ص�����������Ӧˮ�������һ��ǿ����ʣ���a�����ǣ�������
a��b��c��d��e�������ʾ�����һ����ͬ��Ԫ�أ���һ�������¿ɷ�����ͼ��ʾת��������a�ǵ��ʣ�b��ͨ��״���������壬c��d���������e�Ǹ�Ԫ�ص�����������Ӧˮ�������һ��ǿ����ʣ���a�����ǣ�������| A�� | C12 | B�� | N2 | C�� | S | D�� | C |
| A�� | 100mL 1mol•L-1FeCl3��Һ�к���������Ϊ0.1NA | |
| B�� | ��״���£�2.24L��ȩ��ȫȼ������CO2������Ϊ0.2NA | |
| C�� | 0.6gCaCO3��KHCO3�Ļ����������������Ϊ0.3NA | |
| D�� | 80Ml 10mol•L-1Ũ����������MnO2��Ӧ��ת�Ƶ�����Ϊ0.4NA |
| A�� | ������0.4 mol/L HB��Һ��0.2 mol/L NaOH��Һ�������Ϻ���Һ��pH=3��������Һ������Ũ�ȵĴ�С˳��Ϊ��c ��Na+����c ��B-����c ��H+����c ��OH-�� | |
| B�� | ����ʱ��pH��Ϊ2��CH3COOH��Һ��HCl��Һ��pH��Ϊ12�İ�ˮ��NaOH��Һ��������Һ����ˮ�����c��H+����� | |
| C�� | ������0.1 mol/L��������Һ ��NH4Al��SO4��2����NH4Cl����NH3•H2O����CH3COONH4��c ��NH$_4^+$���ɴ�С��˳���ǣ��ڣ��٣��ܣ��� | |
| D�� | 0.1 mol/L NaHB��Һ����pHΪ4��c��HB-����c��H2B����c��B$_{\;}^{2-}$�� |
| A�� | �ŵ�ʱÿת��3 mol���ӣ�������1mol K2FeO4������ | |
| B�� | ���ʱ������ӦΪ��Fe��OH��3-3e-+5OH-=FeO42-+4H2O | |
| C�� | �ŵ�ʱпʧȥ���ӣ�����������Ӧ | |
| D�� | �ŵ�ʱ����������Һ�ļ��Խ��� |
| A�� | ������Ӧ����������ֻ��N2 | |
| B�� | ���ⶨ��NaCN��ˮ��Һ�ʼ��ԣ�˵��CN-�ܴٽ�ˮ�ĵ��� | |
| C�� | ��������Ӧ����0.4 mol CO2������Һ�����������ӵ����ʵ���Ϊ2mol | |
| D�� | ��ȡ1 L��CN-1.02 mg/L�ķ�ˮ��������Ҫ4.0��10-5mol ����������ŷ����ŷű� |
 ��Eԭ�ӵļ۵����Ų�ʽΪ3s23p5��F�����ڱ��е�λ���ǵ������ڵ�IVB�壮
��Eԭ�ӵļ۵����Ų�ʽΪ3s23p5��F�����ڱ��е�λ���ǵ������ڵ�IVB�壮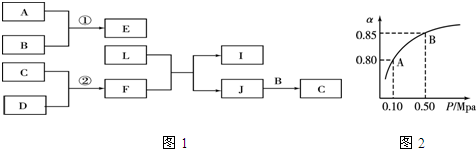
 ��J�Ľṹʽ
��J�Ľṹʽ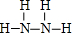 ��
�� ijʡ2005�깤ҵ����Ҫ���ȵ糧�����������ŷ�����Ϊ1.24��106 t��ȫʡ������Ⱦ�Ƚ����أ���������ɷַ��֣���ˮ���������������������Ϊ����Լռ������������61.9%����������笠�����Ϊ����Լռ������������84.1%����������SO42-��NO3-��������Ϊ4.13��1��NO3-�ı������������������ƣ��ش��������⣺
ijʡ2005�깤ҵ����Ҫ���ȵ糧�����������ŷ�����Ϊ1.24��106 t��ȫʡ������Ⱦ�Ƚ����أ���������ɷַ��֣���ˮ���������������������Ϊ����Լռ������������61.9%����������笠�����Ϊ����Լռ������������84.1%����������SO42-��NO3-��������Ϊ4.13��1��NO3-�ı������������������ƣ��ش��������⣺